INTRODUCTION
Survival benchmarking is fundamental in cancer management. First, the survival of spine metastases of lung cancer is highly diverse as it is affected by age, functional status, molecular profiles such as epidermal growth factor receptor (EGFR) mutation, and many difficult-to-measure variables. When the survival outcome of this highly diverse population is derived from a small number of cases, the likelihood of misleading conclusions can be high. Zairi et al. [
1] studied 53 surgical patients with lung cancer with spine metastasis and concluded that surgery is not recommended on account of short life expectancy and frailty. Although employing a large sample size would potentially avoid this, small sample size studies such as this are common in the current literature. One potential solution is to use data from the Global Cancer Observatory [
2], which provides large-scale survival data for lung cancer that inform cancer control, research, and survival benchmarking. Although lung cancer data are available in this format, data regarding patients with spine metastases are not. This defect should be filled in the current era of big data-oriented cancer statistics.
Second, surgeons typically consider fixation or fusion instrumentation appropriate for patients who survive for longer than 1 year after surgical treatment. Wide excision is also considered for patients who survive longer. However, decompression alone or vertebroplasty can be used as alternative treatments for patients with limited survival. Therefore, when treating patients with recurrent lesions after local treatment, surgeons and radio-oncologists need expected survival data for treatment decision-making.
Furthermore, the influence of time is significant and includes progressing aging demographics and the advancement of multimodal oncological treatments. Therefore, survival is not constant but could subtly improve over time. Following the introduction of targeted therapies (such as TKIs) in early 2000, the survival rates of patients with lung cancer have gradually improved [
2]. Accordingly, improved survival is also expected among patients with spinal metastasis; however, this assumption has not been substantiated. The Global Spinal Tumour Study Group attempted—but failed—to statistically demonstrate improved survival among patients with spinal metastasis of lung cancer [
3]. Although this multinational collaborative study included 263 patients, the confidence intervals of the survival curves were wide, and statistically significant differences could not be established (
Fig. 1).
One solution to the above problem is a meta-analysis, which can help obtain a survival function based on large-scale data. Two types of patient populations need to be addressed separately: those who underwent surgery and those who received nonsurgical treatments. In a randomized study conducted in 2005, Patchell et al. [
4] reported that surgery provided a clear advantage in metastatic epidural spinal cord compression. Since then, surgery has become the first option for patients who can physically tolerate it. Moreover, the surgical community seems to fully accept this conclusion, as no other randomized studies have been reported in this field since the study was published [
4]. However, patients who are unlikely to tolerate surgery are considered to have a poorer baseline condition, regardless of cord compression or spinal instability. Therefore, the surgical treatment itself may act as a selection criterion that differentiates the baseline condition. To separately accommodate the 2 types of patients, the current study meta-analyzed each group separately. This review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 guidelines [
5].
MATERIALS AND METHODS
All data are provided within the article and are freely available from the Open Science Framework [
6]. The protocol was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42021256577) on July 8, 2021.
This single-arm meta-analysis analyzed the survival function of non-small cell lung cancer (NSCLC) patients with spinal metastasis. Pathologies other than NSCLC and patients without spinal metastasis were excluded. The patient population was divided into 3 groups: surgery, nonsurgery, and mixed. Surgery was defined as patients undergoing operative treatment such as palliative decompression, debulking, total vertebrectomy, separation surgery, or any form of minimally invasive surgery. Nonsurgery was defined as treatments such as radiotherapy, vertebroplasty, chemotherapy, or palliative care. The mixed category contained combined survival data of surgical and nonsurgical patients. Only studies with extractable survival data were included. Studies with unextractable data, those that included nonNSCLC pathologies or nonspinal metastases, and those that did not report the total number of events were excluded. The context was restricted to clinical settings such as tertiary institutions, medical centers, and oncological centers. There were no restrictions on the type of study (randomized controlled trials or nonrandomized studies) as long as the data were extractable.
1. Finding and Assessing Individual Studies
We searched MEDLINE, Embase, and Google Scholar, as illustrated in
Supplementary material 1. We also included the survival data of 172 patients from our institution (the Spine Oncology Registry of National Taiwan University Hospital). The search strategy for each database is detailed in
Supplementary material 2. The search was performed on June 26, 2021. There were no restrictions on language. Study titles, abstracts, and full text were independently screened for inclusion by 2 authors (CLC and FYT), and discrepancies were resolved based on a consensus with a third author (JPJ). To facilitate transparency and reproducibility, we used a prepiloted form (
Supplementary material 3) in the process of study inclusion and data extraction. No automation tool was used.
For qualitative data, the bias in the body of evidence was assessed according to the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) version dedic ated to prognostic factors [
7]. As the current GRADE framework has no dedicated version to accommodate single-arm reviews, we narrowed the definitions according to the fundamental intention of the assessment (
Supplementary material 4). The risk of bias (study limitations) in individual studies was assessed according to the respective GRADE items. All 3 outcomes were determined
a priori, as presented in a summary of findings table. The GRADE level was independently judged by CLC and FYT, and discrepancies were resolved based on a consensus with JPJ.
2. Synthesizing the Body of Evidence
Data extraction was straightforward for studies that provided the survival data of each patient within the publication. For studies that provided a Kaplan-Meier curve, we used WebPlotDigitizer [
8] to obtain the approximate coordinates in the image. The coordinates were processed using the
IPDfromKM package [
9] in R statistical software (R Development Core Team 2020,
www.rproject.org) to obtain the survival data of each patient. Survival time was measured in months.
The survival data of individuals were pooled to estimate the survival function separately for the surgery, nonsurgery, and mixed groups. The data were also grouped chronologically to facilitate the assessment of changes over time. However, we did not predetermine the exact date of the partition. Moderator analysis was applied to explore the sources of statistical heterogeneity in study-level covariates, with median survival as the effect size. The 5 predetermined study-level covariates were as follows: mean age; starting date of study/subgroup inclusion; surgery or alternative treatment; and proportions of patients who received tyrosine kinase inhibitor (TKI), had an EGFR mutation, or had synchronous/metachronous malignancies. Conventional definitions were used for the confidence interval (95% confidence interval [CI]), heterogeneity (I2< 40%: unimportant; 30%–60%: moderate; 50%–90%: substantial, 75%–100%: considerable), and p-value (<0.05: significant). The online GRADEpro GDT software was utilized for GRADE assessment. The R packages survival, survminer, metafor, and metaCART were used for analysis.
RESULTS
A total of 2,244 studies were identified and screened (
Supplementary material 1), of which 196 studies were assessed for eligibility and 62 were included in the meta-analysis. We documented the reasons for excluding 134 eligible studies (
Supplementary material 5). Three studies were exclusively obtained from Google Scholar, one from the National Taiwan University Hospital Spine Oncology Registry, and the remaining 58 from either MEDLINE or Embase. The characteristics of included studies, including individual citations, are detailed in
Supplementary material 6. The interreviewer agreement using Cohen kappa was 0.927 (96.9% agreement;
Supplementary material 7). Among the included studies, one [
10] was in Chinese, one [
11] in German, and one [
12] in Japanese; the rest were in English.
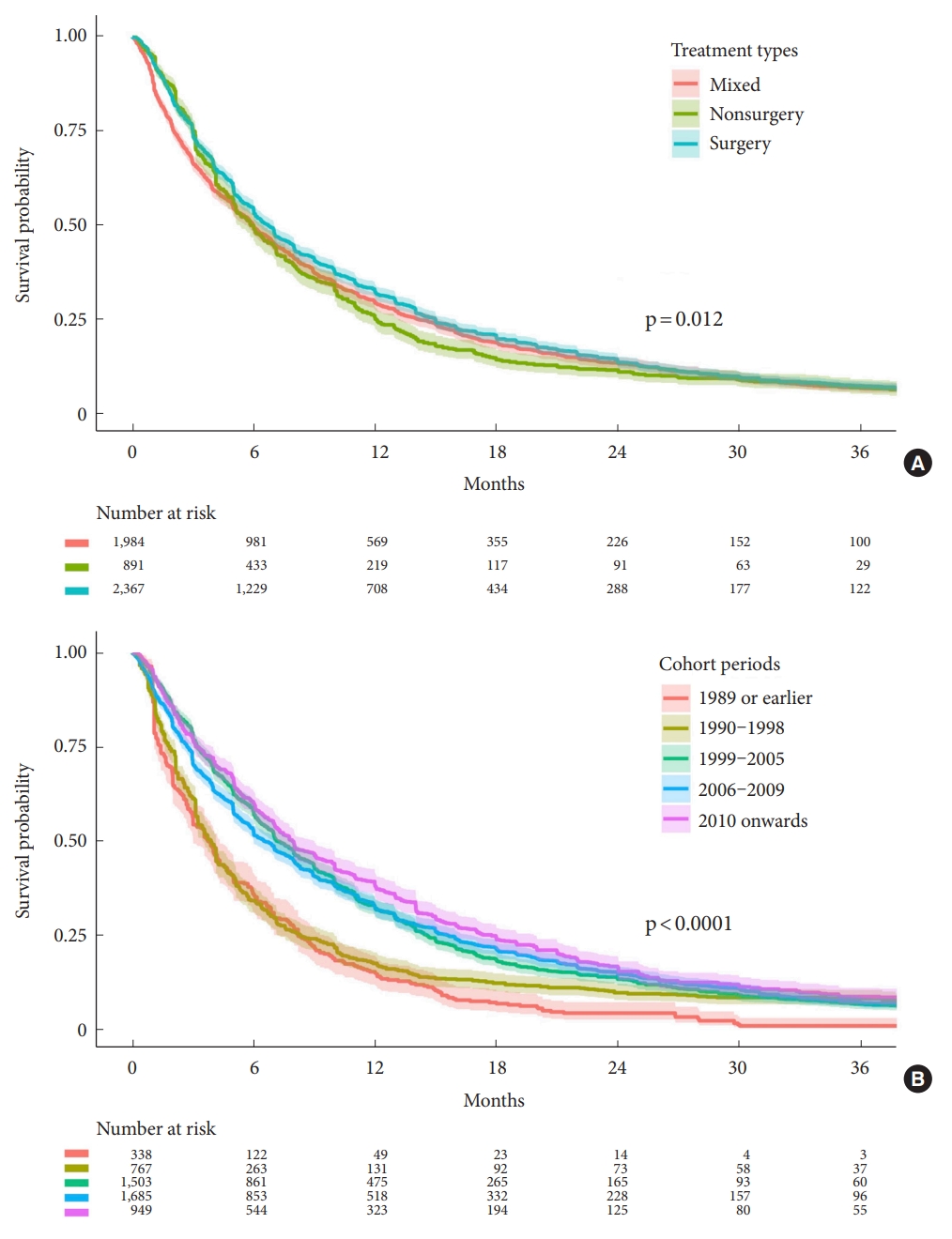
1. Survival Between Treatment Types
The summary of findings table and the pooled survival function of the 3 outcomes are shown in
Table 1 and
Fig. 2A, respectively. The pooled survival function estimated from the data of multiple studies (
Fig. 2A) showed a median survival time of 6.72 months in the surgery group (95% CI, 61.9–7.01; 2,367 participants; 36 studies), 5.99 months in the nonsurgery group (95% CI, 5.33–6.47; 891 participants; 12 studies), and 5.96 months in the mixed group (95% CI, 5.67–6.43; 1,984 participants; 18 studies).
The large-scale survival data narrowed the CI significantly compared to those shown in
Fig. 1. Sections of the curves where the CIs did not overlap suggested a meaningful difference in the survival times of patients between the surgery and nonsurgery groups. The nonsurgery group showed a lower survival probability in the period between 6 months and 30 months. Although a log-rank test indicated significant differences (p = 0.012) between the surgery, nonsurgery, and mixed, the curves began to merge at 30 months and overlapped significantly thereafter (
Fig. 2A).
2. Survival Changes Over Time
Although the study protocol was designed to allow the analysis of potential survival differences over time, the data classification was not predetermined. Therefore, we partitioned the data
a posteriori so as to evenly distribute the number of studies into 5 groups (
Fig. 2B). The partition in 1999 was determined based on moderator analysis, as discussed below. Because the studies had been conducted across varying time spans, a clean partition was impossible. As a compromise, the data were sorted in ascending order according to the time of initial recruitment in each study. We admit that this is an
a posteriori classification. However, the partitions can be further investigated and refined, as the data are freely available from the Open Science Framework [
6].
There were several findings from this analysis. First, patients enrolled since 2010 had the best survival, with a nonoverlapping 95% CI. Second, the partition in 1999 was an output of the moderator analysis, which showed a large survival gap when data were separated. Third, when patients enrolled in 1989 or earlier were omitted, survival times improved in the period before 30 months. Beginning at the 30-month timepoint, all survival curves merged, and the survival probability ranged from 8.5% (95% CI, 6.74%–10.79%) to 11.7% (95% CI, 9.63%–14.15%). The 30-month survival has remained seemingly constant for the recent 2 decades. Finally, starting in 1999, more studies reported TKI usage or EGFR mutation status (see
Supplementary material 8).
3. Moderator Analysis
Along with the 5 study-level covariates mentioned in the Methods section, we also accounted for the exclusiveness of NSCLC. At the stage of study inclusion, 34 studies reported the pathology as NSCLC. However, the pathology was unavailable (i.e., labeled as “lung cancer”) in 36 studies. After discussion, we decided to include both types of studies and perform moderator analysis for this covariate. We divided studies into subgroups to accommodate the study-level covariates. For example, one [
13] of the included studies with an ID of “Yang SZ 2017” reported 2 sets of survival data: surgery and nonsurgery. These were separated into 2 subgroups in the moderator analysis: “Yang SZ 2017-su,” and “Yang SZ 2017-no,” respectively. The subgroups and extracted covariates of all studies are listed in
Supplementary material 6. The
metaCART [
14] package was used to identify the point of partition that best explained the heterogeneity (see
Supplementary material 9). The covariate of synchronous metastasis was not influential according to the analysis in
metaCART. The influential covariates were further visualized with survival data in
Fig. 3. Overall, 5 moderators (excluding the percentage of patients receiving TKIs,
Fig. 3F) were significantly related to survival differences among groups.
The R
2 value indicates how well our separation model explains heterogeneity. As in regression analysis, R
2 values closer to 1 indicate a good model. Although all 6 separation models indicated a significant difference, the R
2 values were low (see
Supplementary material 9). Thus, the analysis accounted for heterogeneity to only a limited extent, and most heterogeneity remained unexplained (residual heterogeneity). This suggests that there was serious heterogeneity in the survival data included in this review (in terms of median overall survival). Although median survival represents only one timepoint (that of 50% survival) in the survival function, this was a significant finding that downgraded several GRADE items, as discussed in the next section.
4. GRADE Assessment
The GRADE scores of all outcomes indicated very low certainty of evidence (
Table 1 and
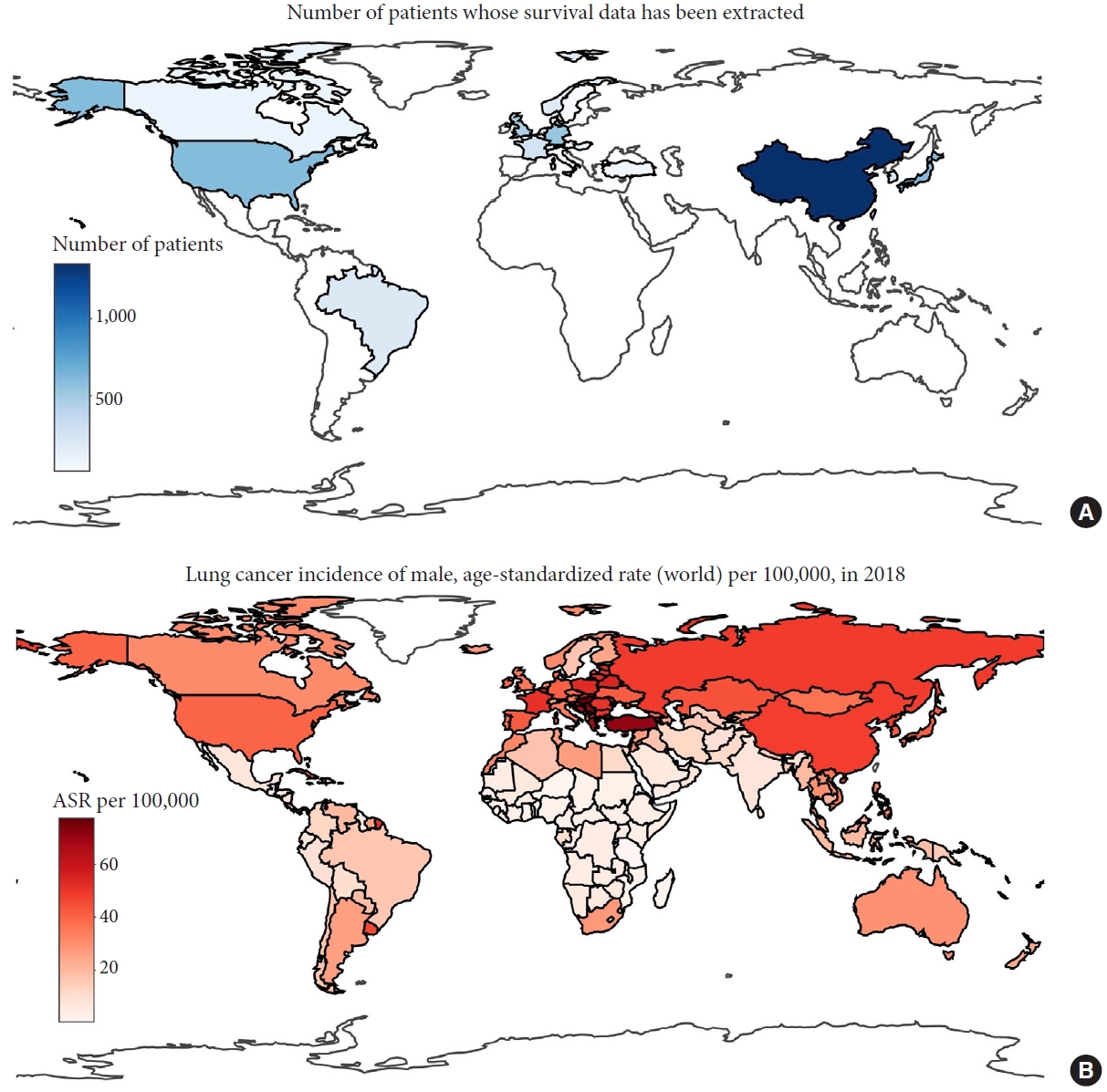
Supplementary material 4). Three main issues are summarized here. First, there was considerable heterogeneity in the data in terms of median survival. Second, studies that reported a single survival curve for surgical and nonsurgical treatments (i.e., those in the mixed category) seldom indicated their inclusion criteria or clinical severity. This can cause sampling discrepancies or bias between studies and make it difficult to define the target population, thus limiting the generalizability of outcomes. However, this issue was less severe in studies in the surgery and nonsurgery categories. Third, we considered studies from a small number of countries or regions, representing a minority of lung cancer cases (according to data from The Cancer Atlas [
15]). This may have introduced publication bias (
Fig. 4).
DISCUSSION
There have been significant advancements in the treatment of lung cancer with spinal metastasis in the past decade. Biomarker-directed therapies, like Osimertinib treatment, are among the newer TKIs therapies replacing previous targeted therapies [
16]. In addition, studies of PD-1 and PD-L1 inhibitors have led to the increased use of immunotherapy with pembrolizumab since 2015 [
16]. Stereotactic radiosurgery is regarded as a game-changer for spinal metastasis in other pathologies, with ongoing trials investigating different sessions [
17]. Radiosurgery has also led to the development of minimally invasive procedures like separation surgery [
18]. Despite these advancements, the durability of these treatments is not ideal [
16]. Hence, it is crucial to determine whether they translate to survival benefits, especially in patients with spinal metastasis.
1. Survival Benchmarking Is Now Possible
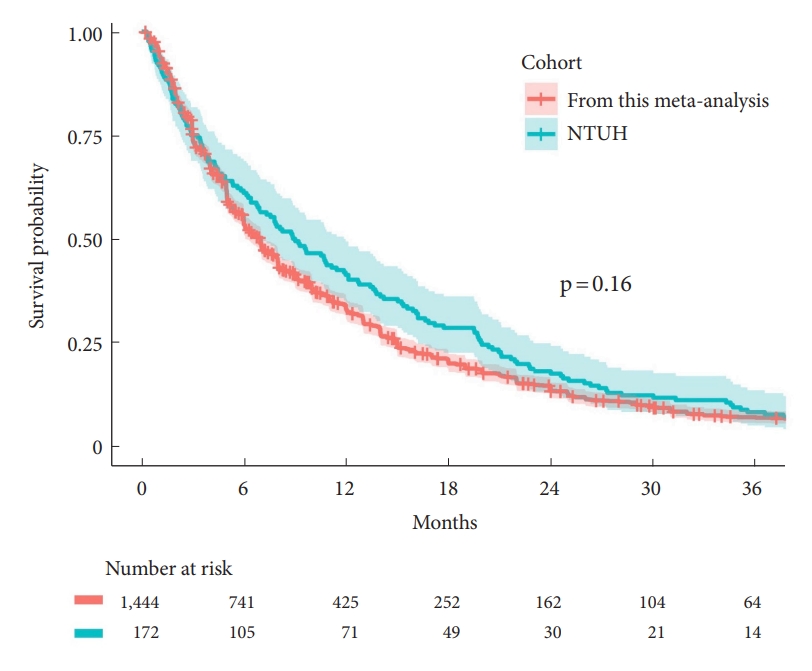
To date, this is the first and the largest dataset representing a collection of all currently published survival. The dataset is open source and is available in the Open Science Framework [
6]. This allows researchers to subset the dataset to complement any individual comparison. To demonstrate this, we compared the survival rates of the surgical cohort of the National Taiwan University Hospital Spine Oncology Registry against those of the dataset. For a meaningful comparison, we subset the dataset that underwent surgery beginning in 2004 (
Fig. 5). A log-rank test indicated a p-value of 0.16, suggesting a similarity to those of the dataset. Furthermore, as described in the Introduction, fusion is typically considered appropriate for patients who survive for longer than 1 year. The 1-year survival of our institute was 41.3%, slightly higher than those of the (subset) dataset of 32.9%. This suggests fusion should be highly considered. Additionally, the high heterogeneity of our dataset reasonably reflected the high diversity of this group of patients. Hence, researchers need to utilize the covariates highlighted in the moderator analysis (
Fig. 3) to subset the appropriate dataset to achieve a fair and meaningful comparison.
For a highly diverse population, benchmarking with a large-scale dataset can avoid misleading conclusions, as described in the Introduction. For instance, Zairi et al. [
1] studied 53 surgical patients with lung cancer with spine metastasis and concluded that surgery is not recommended, on account of short life expectancy and frailty. Although our data suggest that the outcome of these patients is poor, it was not as poor as that described by Zairi et al. [
1]. Frequently, the survival outcome deriving from a small number of cases of highly diverse population results in a Kaplan-Meier curve with large ladder steps and a wide 95% CI. Moreover, we found that the survival of surgical patients was slightly better than those of nonsurgical patients and was statistically significant (p = 0.0046) (
Fig. 3D), although each was meta-analyzed separately.
2. Survival Changes Over Time
Upon analyzing survival changes over time (
Fig. 2B), data from patients enrolled since 2010 had the best survival. This finding could suggest that the overall improvement of survival in lung cancer, in general, also applies to those with spinal metastasis. Although this is expected, it has not previously been presented in statistical terms. Since the data from 2010 may more accurately reflect current survival, researchers should focus on this subset in future benchmarking and remain optimistic in the management of these patients.
However, we also found that improvements were evident only prior to 30 months. The data visualization suggests a time limit of 30 months for survival analysis using this dataset, although this interpretation is not based on any statistical calculations. Our analysis also suggests that future research on comparative effectiveness should aim to reach the minimal period of 30-month follow-up before concluding superiority. On the contrary, studies that use a point estimate, commonly in conjunction with a median survival time, would be unlikely to identify the 30-month impasse. Rothrock et al. [
19] demonstrated a gradual improvement with regression of median survival time in 309 lung cancer patients from 1998 to 2017. Although these findings are consistent with this study (
Fig. 2B) in terms of median survival time, the improvement in outcome at 30 months was not demonstrated. This is because the median survival only serves as a point estimate of the full survival curve and does not encapsulate trends in the first and third quartiles or at any other points of interest. Overall, due to the
a posteriori classification of data, these findings can only be considered exploratory.
3. Limitations
There were significant limitations to this study. First, for survival comparisons between surgery and other groups, any causative conclusions (such as those commonly derived in comparative effectiveness research) would be inappropriate. This is because the survival data were derived from single-arm rather than double-arm trials. Although the grouped data can be illustrated side-by-side, we cannot conclude that either has a relative survival advantage. Second, there was serious heterogeneity in the data. As there is no standard technique for the assessment of heterogeneity in non-parametric survival data, we used median survival as an effect size for heterogeneity assessment; moderator analysis only accounted for a small amount of heterogeneity. Third, the findings related to survival changes over time can only be regarded as exploratory. Studies varied significantly in their follow-up durations, resulting in significant overlaps at each time span when survival data were obtained (
Supplementary material 8). The sorting and partitioning of survival data (
Fig. 2B) was an
a posteriori attempt. Overall, this resulted in very low certainty of evidence, as assessed under the GRADE framework.
CONCLUSION
This is the first large-scale data regarding lung cancer with spinal metastasis that allows survival benchmarking. Data from patients enrolled since 2010 had the best survival and may more accurately reflect current survival. Researchers should focus on this subset in future benchmarking and remain optimistic in the management of these patients.