|
 |
- Search
|
|
||
Abstract
Objective
The aim of this study was to emphasize on the interaction of spatial and temporal gait parameters and analyse the gait asymmetry in the patients with lumbar disc herniation (LDH) before and after microdiscectomy.
Methods
This was a prospective, observational study conducted on 59 cases of LDH planned for lumbar microdiscectomy, and healthy control group with 54 participants for analysis was performed prior to surgery and 15 days after surgery. The spatiotemporal gait parameters were measured using a “Win-Track” gait analysis platform system. All the participants walked barefoot for 10 times with their normal walking speed in the same day. The 3 flawless walking data were recorded and the arithmetic means were computed. The gait symmetry index was used to calculate the walking asymmetry. The pain intensity of the patients was recorded shortly before performing the analysis by a visual analogue scale.
Results
In the postoperative assessment LDH patients had significantly shorter temporal parameters, longer spatial parameters, faster walking speed, and more cadence than the preoperative assessment (p < 0.05). There were improvements in the asymmetry values of the postoperative gait parameters compared to the preoperative values, but these differences were not significant (p > 0.05). In addition, there was a significant difference in all parameters in terms of gait asymmetry between the postoperative assessment and the healthy controls (p < 0.05).
Disc herniation leads to functional loss and disability in daily life activities and negatively affects the quality of life among lumbar disc herniation (LDH) patients [1,2]. The most important clinical complaints in the LDH patients are lumbar and radiating lower extremity pain, weakness, paresthesia, and numbness [3]. Depending on LDH in sprawling of pain to spine and legs, lower extremity functions, especially walking speed are adversely affected and usually walk slower than their healthy individuals [4]. Although there are differences in symptoms and pain intensity of LDH patients, generally inadequate and abnormal gait patterns and thus deterioration in gait quality and capacity occur [5,6].
Gait analysis is a clinically important biomarker for evaluation and treatment planning of disease states [6]. The gait parameters that obtained by gait analysis systems are important gait variables, because they can be easily evaluated and measured to obtain gait deviations and walking difficulties, make a diagnosis, determine appropriate therapy, monitor the patient’s progress, determine the prognosis, and they can help in understanding the functional limitations during gait. The most common parameters selected for gait analysis are the spatiotemporal parameters, which include step duration, swing duration, double support duration, step length, stride length, walking speed (velocity), and cadence [7,8]. Gait symmetry is defined as a coordinated and consistent activity of the lower extremities during walking. In addition, gait asymmetry reflects a natural functional difference between the limbs due to pathology, and is clinically important in measuring gait pattern changes for observe alterations in gait and evaluate rehabilitative intervention effects [9,10]. Studies supports existing recommendations that the gait symmetry index should be used as the most precision assessment of gait symmetry on the basis of spatiotemporal gait parameters [11].
Lumbar microdiscectomy is the most commonly performed minimally invasive spinal surgical procedure for the treatment of patients with LDH, and because it results in less tissue destruction, it minimizes the patient's activity restriction period [12-14]. After lumbar microdiscectomy, patients typically mobilize the surgical day and are discharged home the following day [15]. It is known that increases in walking time in the days after lumbar surgery are one of several factors of major improvement in physical function [16]. In the literature, some new research suggests that 2 weeks of activity restriction following lumbar microdiscectomy may be sufficient for most patients [17]. However, there are no studies on the interactions in the spatiotemporal parameters of gait and the changes in gait asymmetry during this process.
Studies have identified the use of gait parameters as a relevant tool for the evaluation of patients with spinal problems and for objective measurement of outcome and recovery [18]. Although it has been demonstrated that there is a strong relationship between gait parameters and functional disability, especially in patients with LDH, data on the evaluation and use of these parameters for postoperative recovery after microdiscectomy seem to be lacking [19].
The aim of this study was to emphasize on that the interaction of spatiotemporal gait parameters and changes occurring compared to the healthy control group is given importance in the patients with LDH. In addition, this study was planned to analyze the changes in spatiotemporal gait parameters and gait asymmetry in the LDH patients before and 15 days after microdiscectomy within the period of minimum activity restriction. We hypothesized that there would be changes in the spatiotemporal gait parameters and gait asymmetry during the period of minimal activity restriction with the reduction of pain after microdiscectomy.
This study included the patients admitted to Bahçeşehir University Göztepe Medical Park Hospital, Brain and Spine Surgery Department, and were diagnosed with LDH between May 2016 and December 2017. This study and its procedures were approved by the ethical committee of the Bahçeşehir University Faculty of Medicine (Protocol No. 22481095-020-482, 2016-04/07), and it was performed in terms of the guidelines of the Declaration of Helsinki. All patients provided written informed consent.
Also, the following inclusion criteria were applied: (1) individuals who were diagnosed with LDH by a specialist; (2) the patients with LDH had a medical history and proven by MRI; (3) to be diagnosed with disc herniation at L4–5 and L5–S1 levels; (4) aged between 25–80 years old; (5) pain symptom on lumbar area or lower extremity for at least 1 month. Moreover, the exclusion criteria were as follows: (1) receiving any conservative treatment; (2) having congenital deformity in the spine or lower extremity; (3) history of spinal surgery or other diseases affecting gait; (4) pregnancy; (5) situations that may cause balance problems; (6) presence of nerve root damage symptoms; and (7) using assistive gait appliance.
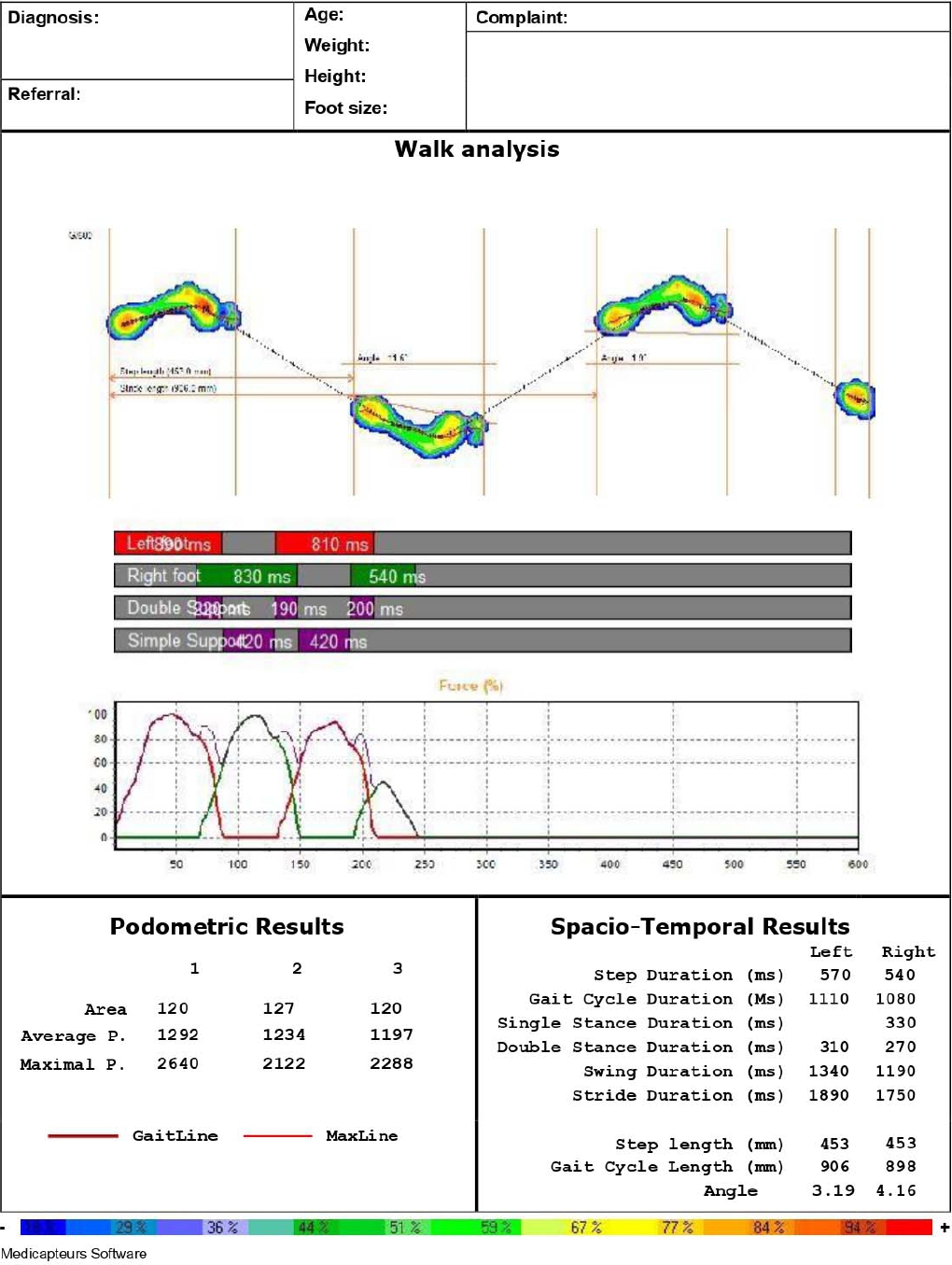
In the present study, quantitative gait assessment was performed using a gait analysis platform system (Win-Track; Medicapteurs, Balma, France). The dimensions of this device are 1,610 mm× 652 mm× 30 mm (length× width× height), the thickness of the platform is 9 mm, which consists of 12,288 resistive type sensors. The dimensions of these sensors are 7.8× 7.8 mm2, and the acquisition frequency of the apparatus is up to 200 images/sec, which has the ability of digitally recording the pedobarographic and spatiotemporal information of subjects’ gait based on the center of foot pressure. The Win-Track platform device is equipped with sensitive sensors that detect all walks and convert them into numerical data, which are then transferred to the computer environment (Fig. 1). Since Win-Track provides fast and high reliability quantitative analysis, this platform was used in this study [20,21]. The test-retest reliability of the “Win-Track” Platform walk analysis platform system was performed by Ramachandra et al. [22] in 2012. As for the gait protocol used in our study, we adopted the 3-step protocol that has been previously shown to have good reliability, with intraclass correlation coefficient values ranging from 0.75 to 0.90 [22].
The sample size and power analysis were performed using the G*Power (v3.1.9.7, Axel Buchner, Universitat Kiel, Germany) program. The sample size for this study was predicted by calculating the differences of the mean values of back pain intensity (1.7) and leg pain intensity (3.5), as well as the avarage values of standard deviation for back pain intensity (2.7) and leg pain intensity (2.75) between presurgery and 3-month follow-up. These measurements were assessed by the visual analogue scale (VAS) based on the study of Schulte et al. [23] which yielded an effect size of d = 0.6605227. With statistical power 95% and an alpha level of 0.05, it was determined that a minimum of 51 participants was needed for each group.
The participants were asked to continuously walk barefoot for 10 times in their normal walking speed as straight as possible without any gait assist device on the Win-Track platform within the same day, prior to and 15 days after surgery. The pain intensity of the patients was recorded shortly before performing the analysis. There was no change in the pain intensity of the patients during the analysis. Three flawless walking data analysis of spatiotemporal gait parameters were recorded and the arithmetic means were computed with 3 repetitions. In the gait chart transferred from the platform to the computer environment during walking, the 3 most perfect gait data, in which the gait cycle (3 steps) and foot pressure can be clearly seen, were selected for analysis. Whenever they were about to take a rest, the patients were allowed to sit down on a chair. The analysis of the spatiotemporal gait parameters was performed on the healthy control group’s participants by the same investigator using the same equipment and measurement protocol (Fig. 2). The patient provided written informed consent for the publication of her identifiable image included in Fig. 2.
The brief description of the gait parameters used in this article is as follows:
Step duration (sec): the time elapsed in the right or left step length.
Gait cycle duration (sec): the time elapsed in a gait cycle.
Double stance duration (sec): the period of time when both heels are in contact with the ground.
Swing duration (sec): the period of time when the foot under consideration is not in contact with the floor.
Step length (cm): the distance between the heel contact of both feet during walking (Fig. 3).
Gait cycle length (cm): the distance between the 2 heel contacts of the same foot (Fig. 3).
Walking speed (velocity) (cm/sec): walking speed is obtained by multiplying step length by cadence.
Cadence (step/min): the number of steps per unit time.
Pain intensity was obtained using a VAS. VAS is usually a horizontal line, containing a numerical range from 0 (no pain) to 10 (the strongest pain), anchored by word descriptors at each end with “no pain” (score of zero) on the left side and “most severe pain level” or “the worst pain imaginable” (score of 100 [100-mm scale]) on the right side. The patient was asked to mark the line point representing his or her current pain [24].
The gait symmetry index (Robinson formula) was used to calculate the asymmetry between the left and right extremities during walking as a percentage (X: gait parameter; L: parameter of left limb; R: parameter of right limb) [25]. The value of 0% indicates perfect symmetry, while increasing values indicates its asymmetry.
The IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA), was used for the all statistical analysis (SPSS Inc., Chicago, IL, USA). Normality was analyzed using the KolmogorovSmirnov test. Chi-square test was used to compare the gender between surgical group and healthy controls. The Independent t-tests for parametric, or Mann-Whitney U-tests for non-parametric data was used to compare the means between surgical group and healthy controls for demographic characteristics, gait symmetry and all the spatiotemporal gait parameters. The paired sample t-test and Wilcoxon test were used to analyze the spatiotemporal gait values and gait symmetry in the patients with LDH before and 15 days after surgery. A p-value of < 0.05 was considered to indicate a statistically significant difference.
In total, 113 participants were included in this study as 59 patients with LDH (24 females, 35 males) and 54 healthy individuals in the control group (27 females, 27 males). The distribution of disc herniation level in the patients with LDH was as follows: 31 patients (52.5%) at the level of L4–5, and 28 (47.5%) patients at the level of L5–S1. There was no significant difference in the distribution of the disc herniation level in LDH (p= 0.696). The side of the disc herniation was distributed 32–27 (54.2%–45.8%) between right and left in LDH. There were also no significant differences between right and left in terms of disc herniation side (p= 0.515).
The demographic characteristics of the participants are presented in Table 1. There were no significant differences between the 2 groups in terms of age, height, weight, body mass index (BMI), and sex (p> 0.05).
The mean (standard deviation) of the pain intensity score was 5.85 (1.36) (range, 3–8) for the patients with LDH before surgery. LDH patients did not report any pain intensity on the 15th day after surgery. Table 2 shows the distribution of the pain intensity score of the disc herniation level in the patients with LDH before surgery. There was no significant difference in terms of VAS scores between the disc herniation levels in the LDH before surgery (p= 0.959).
The spatiotemporal gait parameters were compared with both extremities between the 2 groups. In the preoperative assessment LDH patients had significantly longer step duration, longer gait cycle duration, longer double stance duration, longer swing duration, shorter step length, shorter gait cycle length, slower velocity and less in cadence than the healthy controls. There were significant differences between the 2 groups in all terms of the spatiotemporal gait parameters (p< 0.05) (Table 3).
In the postoperative assessment LDH patients had significantly shorter temporal parameters and longer spatial parameters than the preoperative (Fig. 4). There were significant differences between preoperative and postoperative assessment in all terms of spatial and temporal gait parameters (p< 0.05) (Table 4).
In addition, boxplots in Fig. 4 show the differences in terms of spatial and temporal gait parameters compare between postoperative and healthy controls. In patients with LDH, the temporal parameters of gait at 15 days after surgery were similar to those of the healthy control, and there was no statistically significant difference between the 2 groups (p > 0.05). However, the difference between the postoperative and healthy control groups in terms of the spatial gait parameters were statistically significant (p< 0.05) (Table 4).
Table 5 shows the gait asymmetry values of the patients with LDH before and 15 days after surgery and the healthy control group. There were improvements in the asymmetry values of the postoperative gait parameters compared to the preoperative values, but these improvements were not statistically significant (p> 0.05). The LDH patients in postoperative assessment had significantly higher gait asymmetry than the healthy controls (p< 0.05).
This study was conducted to evaluate the interactions and alterations in spatiotemporal gait parameters and analyze the gait asymmetry pre- and postoperatively in the individuals with and without LDH. Also, the potential impacts of microdiscectomy on the LDH patients were quantitatively assessed by comparing the gait abnormalities before and after performing the surgical procedure. We observed an increase in the spatial and a decrease in temporal characteristics of the gait in the LDH patients compared to those in normal subjects.
The results of our study showed that there were significant differences in all spatiotemporal parameters of gait between LDH patients (preoperative) and healthy controls, and while improvements in all spatiotemporal gait parameters were significant on the 15th day after microdiscectomy compared to presurgery, these improvements were not achieved in gait asymmetry. Furthermore, our findings revealed that while microdiscectomy significantly improves the temporal parameters of gait, these improvements were not meaningful in terms of spatial parameters and gait asymmetry between LDH and healthy subjects.
As far as we know, the present study is the first to examine the gait symmetry of LDH patients before and 15 days after microdiscectomy, and compare with healthy controls. Although changes were obtained postoperative gait asymmetry scores than the preoperative assessment in patients with LDH, differences were not significant. In addition to, it was determined that there was still more gait asymmetry after surgery currently compared to healthy controls.
Some investigators reported important findings regarding the consideration of psychological factors such as lumbar instability, impaired paraspinal muscle activity and quality, lower extremity muscle weakness, and fear assumptions about pain in addition to physiological factors and pain with walking, when studying spinal disorders [26-28]. The presence of these factors leads to inefficient energy expenditure, fatigue and imbalance in spinal and adversely affects the patient’s quality of life and daily activities such as walking [27-29].
In the literature, several investigators have found some differences in gait kinematics between normal and chronic low back pain (CLBP) cases in nonsurgical cases. For example, Barzilay et al. [30] stated that, healthy controls had faster walking speed with longer step length and higher cadence compared to CLBP counterparts. Also, Bacchini et al. [31] examined the gait behaviors in 9 patients with lumbar stenosis using a 3-dimensional (3D) opto-electronic system, and noticed an increase in the double stance duration and the decreased stride length in these patients. Al-Obaidi et al. [27] studied the pain-related factors affecting gait performance in 31 CLBP and 24 normal cases. Conclusively, it has been demonstrated that, step length, single support time, and walking velocity differed between the 2 groups. CLBP patients tend to walk slowly with shorter step length and shorter single support time, which are mainly attributed to psychological factors such as pain-related fear beliefs, rather than physiological ones. Similarly, Hicks et al. [32] studied gait performance in elderly adults (mean age, ~71.5) with CLBP, and demonstrated that, the patients with CLBP have significantly shorter step length, shorter stride length, greater stance times, and longer periods of double support time, which substantially declines the walking speed in these participants as compared to painfree individuals.
In addition, most spatiotemporal parameters differed between the patients and control subjects. The patients with lumbar spinal stenosis (LSS) had the longer stride duration, shorter stride length, and at slower speed compared to the control group [33]. More recently, Miscusi et al. [28] demonstrated a decreased walking velocity and a shorter step length in the LDH group in comparison to control group, which may be resulted from back pain, lumbar instability, and impaired spinal muscle activity.
In line with our previous work, we found a significant difference in all the spatiotemporal gait parameters between the LDH and healthy control groups [34]. LDH group’s participants had significantly longer step duration, longer gait cycle duration, longer double stance duration, longer swing duration, shorter step length, shorter gait cycle length, slower walking speed, and less in cadence for both extremities as compared with healthy controls, which is attributed to the strong correlation between pain intensity and gait parameters [34]. These results are consistent with previous findings reported by Papadakis et al. [35], and Kim et al. [36].
Regarding our results and clinical observations, the LDH patients often try to alter and adopt their gait patterns to alleviate the level of pain induced in different physical activities. They usually avoid wide trunk, pelvic and hip movements, restrict the relative knee range of motion, and refrain from weight transfer between left and right extremity to avoid exacerbation of the pain, to maintain their balance. This strategy can negatively affect their gait parameters resulting in a shorter step length of these extremities and casus deterioration in gait symmetry.
Several studies attempted to assess the improvements in gait pattern after surgical treatment. Accordingly, Suda et al. [37] have assessed the gait performance in the patients with neurogenic intermittent claudication via a ground reaction force plate. They showed that, gait cycle length, velocity, and also the gait pattern markedly improved in these patients 6-month postsurgery. Loske et al. [33] performed a pre- operation and postoperation gait analysis on the patients with LSS and observed notable improvements in gait variables in these group at 12-month follow-up tests. Meanwhile, faster walking speed, higher cadence, and longer gait cycle length were observed after surgery. In another study, Haddas et al. examined the spatiotemporal gait parameters before and 3 months after surgery in patients with degenerative lumbar spondylolisthesis, and they revealed that significant improvements were observed in terms of increased velocity, decreased step duration, and decreased double stance duration after surgery; However, the same was not current for spatial parameters. Moreover, they reported there was a significant difference in terms of spatiotemporal parameters between 12 weeks after surgery and controls, although most of the mean values returned to normal after surgery [38].
In the current study, we noticed that, gait profile is quite different in the LDH patients before and 15 days after microdiscectomy intervention, which is due to a reduction in lower limb pain, increase in the level of self-confidence and ability of walking, enhancement in the muscle strength and endurance, and in lumbar stability as well. Thanks to the short-term effects of surgical treatment and postoperative rehabilitation programs, which promote the self-confidence along with the functional ability in these patients, the spatial and temporal characteristics of the gait achieved the normal level only by passing 15 days postoperation given that. In addition, although the postoperative group had similar temporal parameters to the healthy control group, they had shorter step length, shorter gait cycle length, slower velocity, and less cadence than the healthy control. However, it should be noted that this period (15 days after surgery) is not sufficient to achieve full recovery in gait parameters. The difference in all parameters might be caused by presence of lower extremity insufficient muscle strength, abnormal gait pattern developed by the patient, and pain.
Although some researchers have been carried out on gait symmetry in other spinal disease, it seems that studies are lacking investigating changes in gait asymmetry before and after microdiscectomy surgery within the period of minimum activity restriction in LDH patients [6]. Loske et al. [33] indicated the LSS patients had higher gait asymmetry than the healthy control subjects, and while the spatiotemporal parameters reached normal values ~10th week after surgery, gait asymmetry reached levels of the healthy controls ~12th month after surgery. Morever, they reported that improvements in gait asymmetry (less asymmetric) were highly associated with pain intensity (greater reduction).
Another study by Natarajan et al. [39] evaluated the gait asymmetry clinically by using wearable devices with the new scoring algorithm they suggested and reported in LDH patients who underwent surgery the gait asymmetry index were higher than the control group, and there was a significant difference between the 2 groups. According to the results of this research, that although the mean values of gait asymmetry were different before and after surgery in patients with LDH, this difference was not significant between the 2 assessments. It also revealed that 15 days after surgery is not a sufficient period to achieve improvements in gait symmetry and regain normal values.
According to the authors clinical experiences there are possible reasons for differences of the gait asymmetry in the LDH patients after surgery compared with the control group. The weak proximal limb, decreased core muscle stability, lower extremity muscle weakness of the affected side and pain intensity and/or perception are among the major causes that are likely to persist. Overall, as it is known that the presence of a unilateral limp and the compensatory limb motions resulted in increased the gait asymmetry factors and reduced smoothness of limb motions in LDH patients.
This study has several strengths, and the most notable one was the sample size, which is large enough and uniform (e.g., similar age ranges and BMI, and use of one type of surgery) to guarantee and credit the reliability and applicability of our findings. Another strength of our research was that, repeated the measurements and gait asymmetry analysis, which is of paramount importance when the end goal is clinical application. Also, it has some limitations such as inaccessibility to 3D camera set and other forms of inquiry and inability to obtain kinetic and kinematic values. Evaluation of physical activity levels of the patients and the fact that the gait habits of everyday life were not evaluated, can be acceptable. We suggest that future studies should investigate when patients with LDH will regain normal gait patterns after the surgical treatment. In addition to, the Win-Track platform system offers distinct advantages over 3D camera systems used in clinics. Specifically, it provides a faster analysis and is more cost-effective. However, we acknowledge that access to this platform may not always be available. In such cases, an alternative approach would be to record the patient’s walking and have it analyzed by a specialist in the field. It is important to note, however, that the results obtained through this method may not be entirely objective.
Regarding the methodology pursued in our study and the objective analysis of the outcomes, it is feasible to achieve the best possible decision given in clinical applications without the need for performing any further analysis.
This paper attempted to assess the spatiotemporal gait and gait symmetry alterations in the LDH patients, then compared the results with healthy control group, and also compared with preoperative and postoperative results. Although the LDH patients develop a unique gait strategy to prevent pain intensity, it negatively affects normal gait parameters and this cause different problems in later periods. In LDH patients, eliminating or minimizing pain with microdiscectomy surgery can improve spatiotemporal gait parameters in the period of minimum activity restriction, but this period seems insufficient to achieve full recovery in gait and regain gait symmetry. For this reason, in order to eliminate deficiencies and provide full recovery, it is necessary to give importance to ambulatory gait training and treatment in rehabilitation programs in the period of minimum activity restriction. Our results can guide the patient-specific evaluating and implementation of gait rehabilitation programs, and design protocols before or after surgery in the LDH patients. The results of this study showed that it is possible to obtain better results in time and distance parameters of walking by relieving or eliminating pain in LDH patients with minimally invasive surgical methods such as microdiscectomy. However, it is important to note that factors such as impaired paraspinal muscle activity and quality, lower extremity muscle weakness and psychological factors that may affect walking, apart from pain, should be considered in the treatment.
NOTES
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: MARB, SS, TKC,IY, DK, ZT; Data curation: MARB, TKC, DK, ZT; Formal analysis: MARB, TKC, MA, IY; Methodology: MARB, SS, TKC, IY, DK, ZT; Project administration: MARB, SS, TKC, IY, DK, ZT; Visualization: MARB, SS, TKC, MA, IY, DK, ZT; Writing - original draft: MARB, SS, TKC, IY, DK, ZT; Writing - review & editing: MARB, SS, TKC, MA, IY, DK, ZT.
ACKNOWLEDGEMENTS
The authors acknowledge Associate Professor Emre Işçi for his invaluable contributions to this paper.
Fig. 4.
Box plot presentation of mean values for spatial and temporal gait parameters before (preoperative), 15 days after surgery (postoperative), and healthy controls group. (A) Step duration, (B) gait cycle duration, (C) double stance duration, (D) swing duration, (E) step length, (F) gait cycle length, (G) velocity, and (H) cadence. *p<0.05. °p>0.05. aPaired sample t-test. bWilcoxon test. cIndependent t-test. dMann-Whitney U-test.
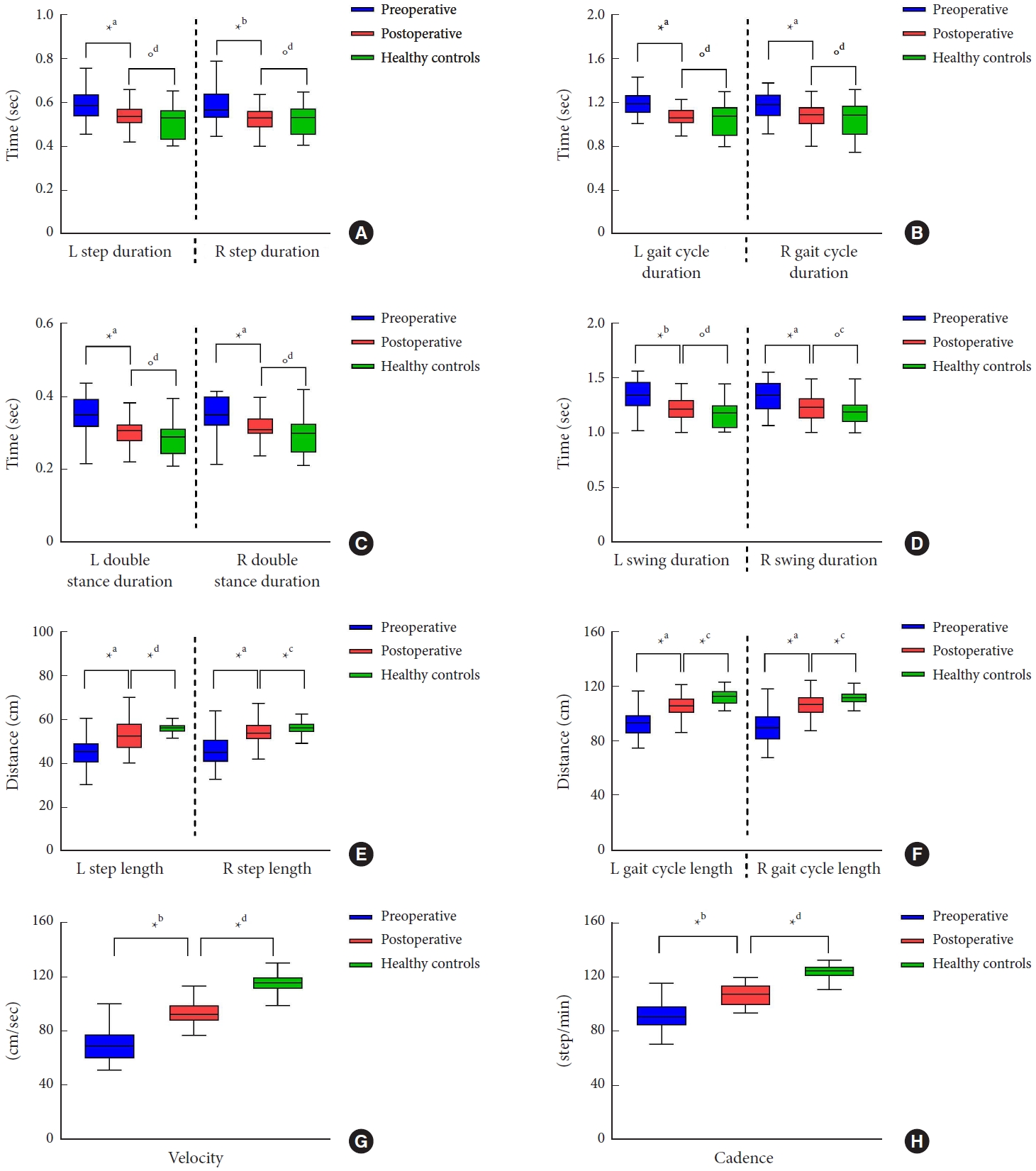
Table 1.
Demographic characteristics of the participants
Table 2.
Pain intensity score of vertebral level before surgery
| No. | Pain intensity | p-value | |
|---|---|---|---|
| Disc herniation level | 0.959 | ||
| L4–5 | 31 | 5.84 ± 1.51 (3–8) | |
| L5–S1 | 28 | 5.86 ± 1.21 (4–8) |
Table 3.
The distribution of the temporal and spatial gait parameters in LDH and healthy controls
| Parameter | LDH preop (n=59) | Healthy controls (n=54) | p-value |
|---|---|---|---|
| L step duration (sec) | 0.59 (0.57–0.61) | 0.52 (0.50–0.56) | < 0.001‡ |
| R step duration (sec) | 0.58 (0.56–0.60) | 0.52 (0.50–0.54) | < 0.001‡ |
| L gait cycle duration (sec) | 1.19 (1.16–1.21) | 1.05 (1.02–1.08) | < 0.001‡ |
| R gait cycle duration (sec) | 1.17 (1.13–1.20) | 1.05 (1.01–1.09) | < 0.001‡ |
| L double stance duration (sec) | 0.35 (0.33–0.36) | 0.29 (0.28–0.30) | < 0.001† |
| R double stance duration (sec) | 0.35 (0.33–0.36) | 0.30 (0.28–0.31) | < 0.001† |
| L swing duration (sec) | 1.35 (1.31–1.38) | 1.18 (1.15–1.22) | < 0.001‡ |
| R swing duration (sec) | 1.34 (1.31–1.37) | 1.20 (1.17–1.23) | < 0.001‡ |
| L step length (cm) | 45.06 (43.57–46.65) | 56.33 (55.68–57.02) | < 0.001† |
| R step length (cm) | 45.65 (43.88–47.53) | 56.58 (55.71–57.50) | < 0.001† |
| L gait cycle length (cm) | 93.23 (90.88–95.93) | 112.70 (111.32–114.13) | < 0.001† |
| R gait cycle length (cm) | 90.69 (87.92–93.77) | 111.75 (110.29–114.13) | < 0.001† |
| Velocity (cm/sec) | 69.87 (67.23–72.87) | 115.70 (113.87–117.38) | < 0.001† |
| Cadence (step/min) | 91.07 (88.57–93.54) | 123.74 (122.30–125.08) | < 0.001† |
Table 4.
Comparison of spatial and temporal gait parameters between preoperative and postoperative, and between preoperative and healthy controls
| Parameter |
LDH (n = 59) |
Within-group change score | p-valuea) | Healthy controls (n = 54) | p-valueb) | |
|---|---|---|---|---|---|---|
| Preoperative | Postoperative | |||||
| L step duration (sec) | 0.59 (0.57–0.61) | 0.54 (0.52–0.55) | -0.05 (-0.08 to -0.03) | < 0.001† | 0.52 (0.50–0.56) | 0.215 |
| R step duration (sec) | 0.58 (0.56–0.60) | 0.52 (0.51–0.54) | -0.06 (-0.09 to -0.04) | < 0.001‡ | 0.52 (0.50–0.54) | 0.993 |
| L gait cycle duration (sec) | 1.19 (1.16–1.21) | 1.07 (1.05–1.09) | -0.11 (-0.15 to -0.09) | < 0.001† | 1.05 (1.02–1.08) | 0.675 |
| R gait cycle duration (sec) | 1.17 (1.13–1.20) | 1.07 (1.05–1.10) | -0.1 (-0.14 to -0.06) | < 0.001† | 1.05 (1.01–1.09) | 0.548 |
| L double stance duration (sec) | 0.35 (0.33–0.36) | 0.30 (0.28–0.31) | -0.05 (-0.07 to -0.03) | < 0.001† | 0.29 (0.28–0.30) | 0.084 |
| R double stance duration (sec) | 0.35 (0.33–0.36) | 0.31 (0.30–0.32) | -0.04 (-0.06 to -0.02) | < 0.001‡ | 0.30 (0.28–0.31) | 0.054 |
| L swing duration (sec) | 1.35 (1.31–1.38) | 1.22 (1.18–1.24) | -0.13 (-0.18 to -0.08) | < 0.001† | 1.18 (1.15–1.22) | 0.113 |
| R swing duration (sec) | 1.34 (1.31–1.37) | 1.22 (1.19–1.25) | -0.12 (-0.16 to -0.08) | < 0.001† | 1.20 (1.17–1.23) | 0.162 |
| L step length (cm) | 45.06 (43.57–46.65) | 52.53 (50.79–54.22) | 7.50 (5.40– 9.60) | < 0.001† | 56.35 (55.68–57.02) | < 0.001ll |
| R step length (cm) | 45.65 (43.88–47.53) | 54.14 (52.68–55.35) | 8.50 (6.50–10.50) | < 0.001† | 56.58 (55.71–57.50) | 0.007§ |
| L gait cycle length (cm) | 93.23 (90.88–95.93) | 106.13 (104.17–108.22) | 13.00 (10.00–16.00) | < 0.001† | 112.71 (111.32–114.13) | < 0.001§ |
| R gait cycle length (cm) | 90.69 (87.92–93.77) | 106.67 (104.73–108.65) | 16.00 (12.30–19.30) | < 0.001† | 111.75 (110.29–114.13) | < 0.001§ |
| Velocity (cm/sec) | 69.87 (67.23–72.87) | 94.61 (92.39–96.90) | 24.73 (21.87–27.51) | < 0.001‡ | 115.70 (113.87–117.38) | < 0.001ll |
| Cadence (step/min) | 91.07 (88.57–93.54) | 106.70 (104.73–108.54) | 15.60 (13.14–18.09) | < 0.001‡ | 123.74 (122.30–125.08) | < 0.001ll |
Table 5.
Results of the gait symmetry in preoperative, postoperative and healthy controls
| Parameter |
LDH (n = 59) |
p-valuea) | Healthy controls (n = 54) | p-valueb) | |
|---|---|---|---|---|---|
| Preoperative | Postoperative | ||||
| Step duration (sec) | 13.34 (10.62–16.23) | 10.63 (8.59–12.98) | 0.135 | 2.22 (1.60–3.06) | < 0.001 |
| Gait cycle duration (sec) | 7.51 (5.84–9.12) | 5.93 (4.59–7.24) | 0.180 | 2.34 (1.57–3.26) | < 0.001 |
| Double stance duration (sec) | 6.74 (4.70–8.85) | 8.64 (6.78–10.86) | 0.236 | 3.31 (2.04–4.97) | 0.001 |
| Swing duration (sec) | 6.32 (4.35–8.73) | 6.68 (5.33–8.12) | 0.784 | 2.42 (1.80–3.32) | < 0.001 |
| Step length (cm) | 12.22 (9.67–14.91) | 9.15 (7.14–11.24) | 0.085 | 3.81 (2.96–4.85) | 0.002 |
| Gait cycle length (cm) | 7.01 (5.45–8.71) | 4.49 (3.29–5.83) | 0.061 | 3.34 (2.42–4.45) | 0.038 |
REFERENCES
2. Jansson A, Nemeth G, Granath F, et al. Health-related quality of life in patients before and after surgery for a herniated lumbar disc. J Bone Joint Surg 2005;87:959-64.


3. Kim K, Isu T, Morimoto D, et al. Common diseases mimicking lumbar disc herniation and their treatment. Mini-invasive Surg 2017;1:43-51.


4. Huang YP, Bruijn SM, Lin JH, et al. Gait adaptations in low back pain patients with lumbar disc herniation: trunk coordination and arm swing. Eur Spine J 2011;20:491-9.



5. Maldaner N, Sosnova M, Zeitlberger AM, et al. Responsiveness of the self-measured 6-minute walking test and the Timed Up and Go test in patients with degenerative lumbar disorders. J Neurosurg Spine 2021;35:52-9.
6. Natarajan P, Fonseka RD, Kim S, et al. Analysing gait patterns in degenerative lumbar spine diseases: a literature review. J Spine Surg 2022;8:139-48.



7. Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture 2003;17:68-74.


8. Garbelotti SA Jr, Lucareli PR, Ramalho A Jr, et al. An investigation of the value of tridimensional kinematic analysis in functional diagnosis of lumbar spinal stenosis. Gait Posture 2014;40:150-3.


9. Viteckova S, Kutilek P, Svoboda Z, et al. Gait symmetry measures: a review of current and prospective methods. Biomed Signal Process Control 2018;42:89-100.

10. Kuligowski T, Sipko T. Lumbopelvic biomechanics in patients with lumbar disc herniation-prospective cohort study. Symmetry 2021;13:602.

11. Błażkiewicz M, Wiszomirska I, Wit A. Comparison of four methods of calculating the symmetry of spatial-temporal parameters of gait. Acta Bioeng Biomech 2014;16:29-35.
12. Parker SL, Xu R, McGirt MJ, et al. Long-term back pain after a single-level discectomy for radiculopathy: incidence and health care cost analysis. J Neurosurg Spine 2010;12:178-82.


13. Daly CD, Lim KZ, Lewis J, et al. Lumbar microdiscectomy and post-operative activity restrictions: a protocol for a single blinded randomised controlled trial. BMC Musculoskelet Disord 2017;18:312.




14. Yuan S, Wu Q, Zang L, et al. Posterior apophyseal ring fracture in adult lumbar disc herniation: an 8-year experience in minimally ınvasive surgical management of 48 cases. Neurospine 2022;19:586-93.




15. Kelly A, Griffith H, Jamjoom A. Results of day-case surgery for lumbar disc prolapse. Br J Neurosurg 1994;8:47-9.


16. Gilmore SJ, Hahne AJ, Davidson M, et al. Predictors of substantial improvement in physical function six months after lumbar surgery: is early post-operative walking important? A prospective cohort study. BMC Musculoskelet Disord 2019;20:418.




17. Bono CM, Leonard DA, Cha TD, et al. The effect of short (2-weeks) versus long (6-weeks) post-operative restrictions following lumbar discectomy: a prospective randomized control trial. Eur Spine J 2017;26:905-12.



18. Mobbs RJ, Mobbs RR, Choy WJ. Proposed objective scoring algorithm for assessment and intervention recovery following surgery for lumbar spinal stenosis based on relevant gait metrics from wearable devices: the Gait Posture index (GPi). J Spine Surg 2019;5:300-9.



19. Zheng CF, Liu YC, Hu YC, et al. Correlations of Japanese Orthopaedic Association Scoring Systems with gait parameters in patients with degenerative spinal diseases. Orthop Surg 2016;8:447-53.




20. Varvarousis DN, Dimopoulos D, Vasileiadis GI, et al. Do gait parameters improve after botulinum toxin injections in post stroke patients? A prospective study. Toxicon 2021;200:189-97.


21. Chenamgere GK, Maiya AG, Manjunath Hande H, et al. Analysis of gait characteristics using a dynamic foot scanner in type 2 diabetes mellitus without peripheral neuropathy. JESP 2015;11:58-64.

22. Ramachandra P, Maiya AG, Kumar P. Test-retest reliability of the Win-Track platform in analyzing the gait parameters and plantar pressures during barefoot walking in healthy adults. Foot Ankle Spec 2012;5:306-12.



23. Schulte TL, Schubert T, Winter C, et al. Step activity monitoring in lumbar stenosis patients undergoing decompressive surgery. Eur Spine J 2010;19:1855-64.




24. Boonstra AM, Schiphorst Preuper HR, Reneman MF, et al. Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int J Rehabil Res 2008;31:165-9.


25. Robinson R, Herzog W, Nigg BM. Use of force platform variables to quantify the effects of chiropractic manipulation on gait symmetry. J Manipulative Physiol Ther 1987;10:172-6.

26. Kim KH. The ımportant role of paraspinal muscle quality for maintaining sagittal balance while walking: commentary on “correlation of paraspinal muscle mass with decompensation of sagittal adult spinal deformity after setting of fatigue post 10-minute walk”. Neurospine 2021;18:504-5.




27. Al-Obaidi SM, Al-Shuwaie N, et al. The influence of pain and pain-related fear and disability beliefs on walking velocity in chronic low back pain. Int J Rehabil Res 2003;26:101-8.


28. Miscusi M, Serrao M, Conte C, et al. Spatial and temporal characteristics of the spine muscles activation during walking in patients with lumbar instability due to degenerative lumbar disk disease: evaluation in pre-surgical setting. Hum Move Sci 2019;66:371-82.

29. Bae J, Sathe A, Lee SM, et al. Correlation of paraspinal muscle mass with decompensation of sagittal adult spinal deformity after setting of fatigue post 10-minute walk. Neurospine 2021;18:495-503.




30. Barzilay Y, Segal G, Lotan R, et al. Patients with chronic nonspecific low back pain who reported reduction in pain and improvement in function also demonstrated an improvement in gait pattern. Eur Spine J 2016;25:2761-6.



31. Bacchini M, Rovacchi C, Rossi M. Biomechanic risk factorsf or patients withlumbar stenosis shown through gait analysis. Gait Posture 2008;28(Supplement 1):S1-2.

32. Hicks GE, Sions JM, Coyle PC, et al. Altered spatiotemporal characteristics of gait in older adults with chronic low back pain. Gait Posture 2017;55:172-6.



33. Loske S, Nuesch C, Byrnes KS, et al. Decompression surgery improves gait quality in patients with symptomatic lumbar spinal stenosis. Spine J 2018;18:2195-204.


34. Bonab M, Colak TK, Toktas ZO, et al. Assessment of spatiotemporal gait parameters in patients with lumbar disc herniation and patients with chronic mechanical low back pain. Turk Neurosurg 2020;30:277-84.

35. Papadakis NC, Christakis DG, Tzagarakis GN, et al. Gait variability measurements in lumbar spinal stenosis patients: part A. Comparison with healthy subjects. Physiol Meas 2009;30:1171-86.


36. Kim HJ, Chun HJ, Han CD, et al. The risk assessment of a fall in patients with lumbar spinal stenosis. Spine (Phila Pa 1976) 2011;36:588-92.

37. Suda Y, Saitou M, Shibasaki K, et al. Gait analysis of patients with neurogenic intermittent claudication. Spine (Phila Pa 1976) 2002;27:2509-13.



- TOOLS
- Related articles in NS
-
Journal Impact Factor 3.2