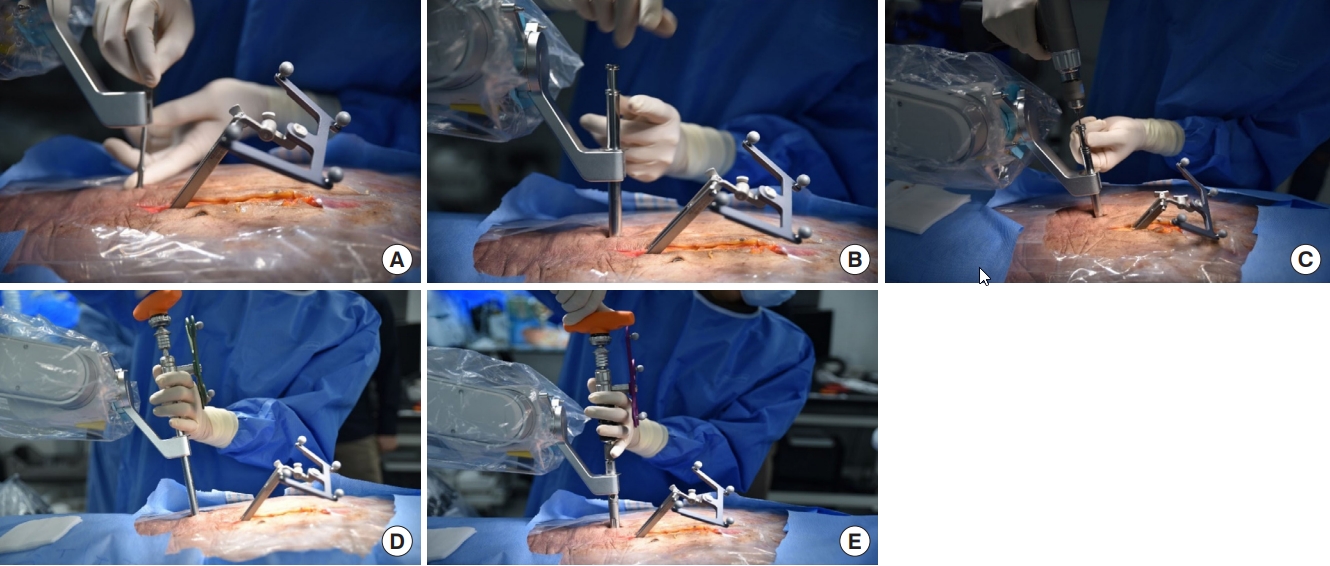
INTRODUCTION
In the field of spine surgery, the precise placement of pedicle screws is crucial. While percutaneous pedicle screw systems have gained popularity in minimally invasive surgery, they can pose risks of radiation exposure to both patients and operating room staff [
1,
2]. It has been reported that fluoroscopically guided pedicle screw insertion in minimally invasive surgery leads to a 2-fold higher dose of radiation compared to pedicle screw insertion in open surgery [
3]. Robotic-assisted surgical systems have been developed to simplify pedicle screw insertion, streamline surgical workflows, reduce operative times, minimize surgical complications, improve clinical outcomes, lower radiation exposure, and potentially decrease costs by reducing hospital stays [
4-
6].
Inserting thoracolumbar pedicle screws is technically demanding, and inaccurate screw placement can lead to serious complications such as neurological, vascular, or visceral injuries [
7]. The rate of screw misplacement ranges from 4.9% to 13.3%, and can increase up to 15.7% when evaluated with computed tomography (CT) scans [
8]. As a result, navigation and robotic-assisted surgery have been developed to enhance accuracy and reduce complications [
5,
9-
12]. Various robotic systems for spine surgery have been developed and tested including SpineAssist, Renaissance, Mazor X (Mazor Robotics Inc., Caesarea, Israel), Mazor X Stealth Edition (Medtronic, Minneapolis, MN, USA), ExcelsiusGPS (Globus Medical, Inc., Audubon, PA, USA), ROSA Spine (Medtech, Montpellier, France), TianJi (Beijing Tinavi Medical Technology Co., Beijing, China), and Cirq (Brainlab, Munich, Germany) [
13-
16]. Previous studies comparing navigated robot-assisted technology with the freehand technique have demonstrated improved accuracy in screw placement and reduced radiation exposure [
9,
10,
17,
18]. However, when measuring the accuracy of robotic-guided pedicle screw placement using 3-dimensional (3D) quantitative methodology, there is still a risk of error [
4].
In our previous studies, we assessed the accuracy of pedicle screw placement using a novel navigation-based spine surgery robotic system in porcine and cadaver models. We confirmed the system’s safety and accuracy in executing planned trajectories and screw path [
19,
20]. Building on the successful results from animal experiments and cadaver studies, we implemented the robot-assisted spine surgery system in clinical practice. Recognizing the higher accuracy and safety requirements in human surgeries compared to animal experiments, we conducted comprehensive verification of the spine surgery robotic system before using it on patients who required precise pedicle screw insertion.
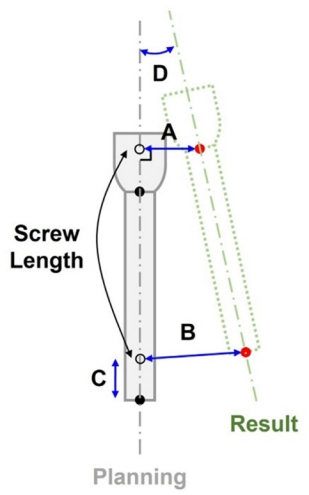
To the best of our knowledge, there have been limited multicenter studies investigating the 3D quantitative accuracy assessment of spine robots. The objective of our multicenter retrospective study was to evaluate the accuracy and safety of robot-assisted spinal surgery in patients, utilizing a unique methodology for calculating 3D offsets.
RESULTS
The results showed that a total of 116 patients underwent robot-assisted spine surgery from 2020 to 2022. Among these cases, 3 were aborted due to technical difficulties, and 113 patients were ultimately enrolled in the study. The average age of the patients was 68.0±7.9 years, with 79 (69.9%) being female. The mean BMI was 24.7±3.4 kg/m2 and the mean BMD was -0.9±1.5. The mean intraoperative estimated blood loss was 392.6 mL and the average length of hospital stay was 9.3 days. Out of the enrolled patients, 5 underwent PLIF with open screw placement under robot assistance, 56 underwent PLIF with percutaneous screw placement, and 52 underwent OLIF with percutaneous screw placement. Among the PLIF cases, 6 patients underwent intraoperative C-arm scan for image registration with the robot system.
A total of 448 screws were placed in the 113 enrolled patients, with an average of 4.2 screws were inserted in 2.2 vertebrae per case. The mean duration of the robot-assisted procedure was 68.9 minutes, and the mean time per screw was 5.9 minutes. Demographic data including surgical parameters are provided in
Table 1. The postoperative CT images were used to assess all inserted pedicle screws. As shown in
Table 2, 448 screws in 113 patients were distributed from T11 to S1, with L4 and L5 accounting for 79.9% of the total. The image registration success rate was 95.16%.
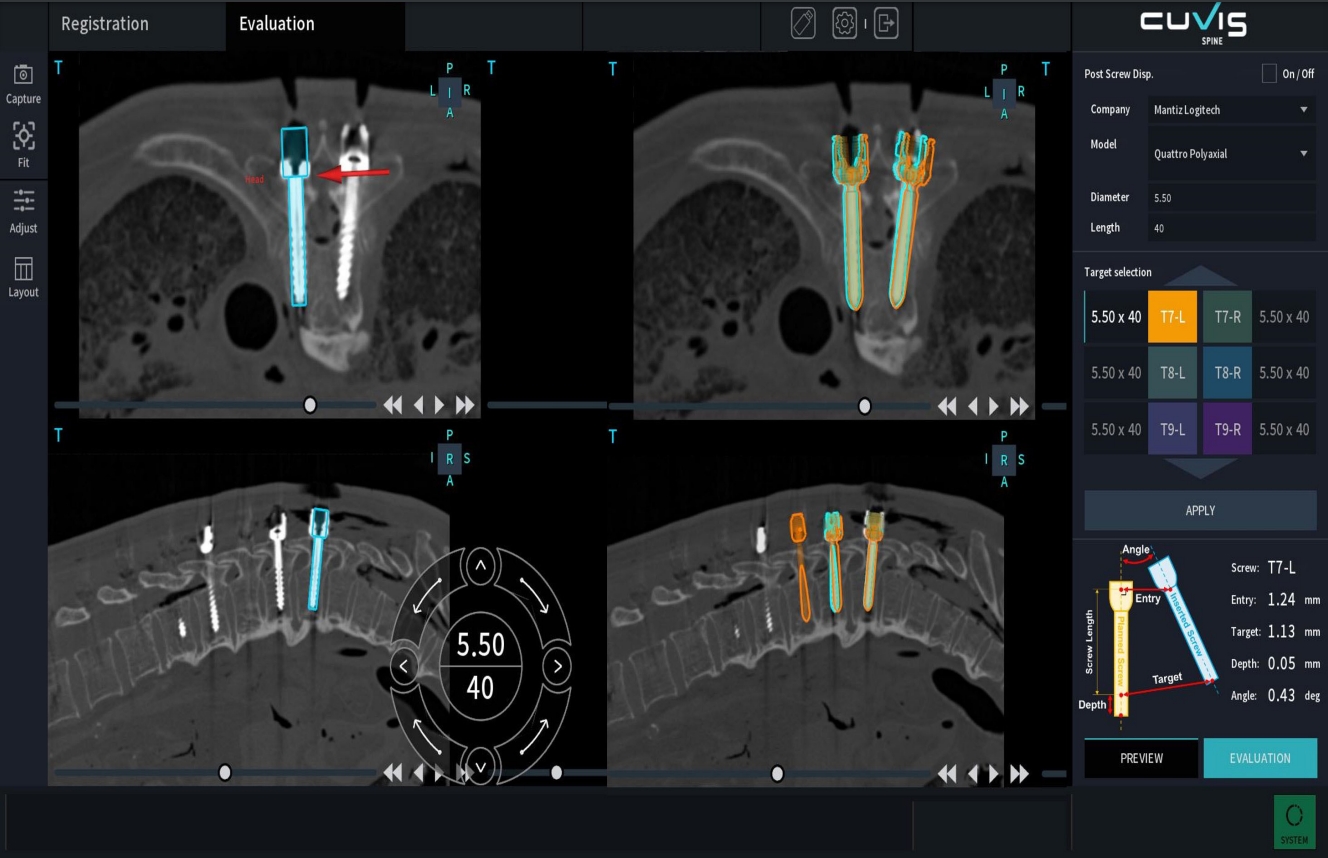
Table 3 presents the descriptive statistics of mean offset values and GRS classification based on the screw insertion technique in the cohort of patients who underwent robot-assisted spinal surgery. A total of 29 screws were inserted using an open approach in the setting of robot assistance and categorized into the open group. The remaining 419 screws were classified as the percutaneous group. The overall mean offsets were as follows: entry offset 2.86±1.64 mm, target offset 2.48±1.74 mm, depth offset 1.99±2.13 mm, and angular offset 3.07±2.31 mm. While the open group exhibited slightly higher mean offsets (entry, target, depth) compared to the percutaneous group, the percutaneous group showed a slightly higher mean angular offset. However, the difference between the groups did not reach statistical significance. According to the GRS grading system, 396 screws (88.39%) were classified as grade A, 43 screws (9.60%) as grade B, 7 screws (1.56%) as grade C, 1 screw (0.22%) as grade D, and 1 screw (0.22%) was grade E. Clinically acceptable screws (GRS grade A or B) accounted for 97.99%. There was a statistically significant difference between the open and the percutaneous groups in terms of GRS grades.
In
Table 4, the descriptive statistics and p-values for the type of interbody fusion are presented. The mean entry offset was 2.66±1.36 mm for PLIF and 3.08±1.89 mm for OLIF, with a statistically significant difference (p=0.009). There were no statistically significant differences observed in the mean target offset (p=0.362) and depth offset (p=0.191) between the 2 groups. However, the mean angular offset differed significantly, with PLIF having a mean of 2.33±1.57 mm and OLIF having a mean of 3.87±2.69 mm (p<0.001). Additionally, the GRS grade distribution showed a statistically significant difference between the PLIF and OLIF groups (p=0.023).
Most of the clinically unacceptable screws (GRS grade C, D or E) showed lateral cortical breach (8/9 screws, 88.89%). One screw classified as GRS grade E which was misplaced laterally at right L4 pedicle is shown in (
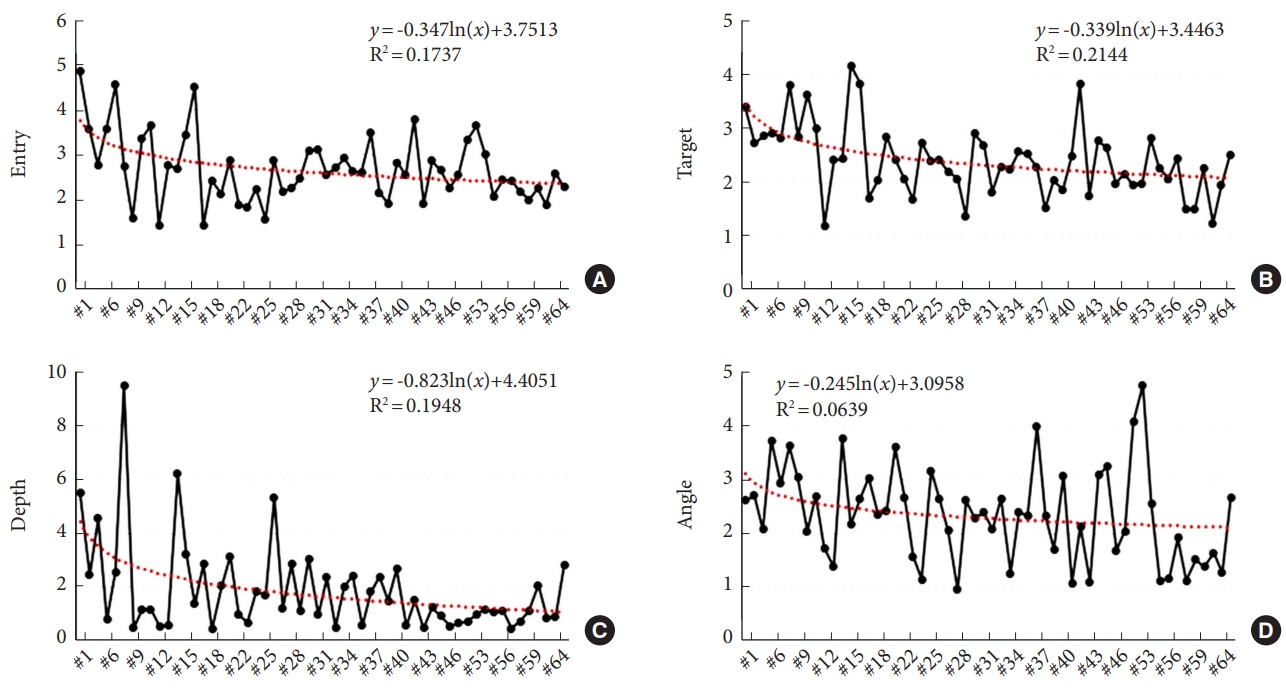
Supplementary ig. 3). No patients experienced iatrogenic neurovascular damage or postoperative infection, and there were no cases of symptomatically misplaced screws requiring revision. The 3D offsets gradually decreased with an increasing number of operation cases (
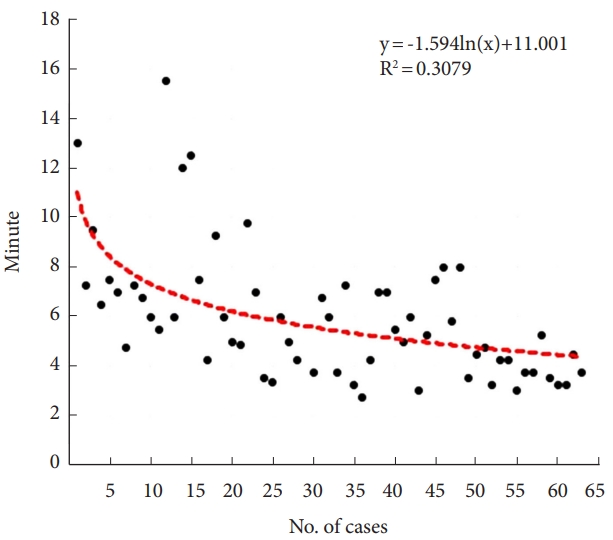
Fig. 5). The time per screw also showed a tendency to decrease with an increasing number of cases (y=-1.594 ln(x) + 11.001, R
2=0.308) (
Fig. 6).
DISCUSSION
According to the previous studies, the conventional techniques used in pedicle screw placement cause screw misplacement, with rates ranging from 3% to 55% [
22]. As precise pedicle screw insertion is one of the most important factors in spine surgery, navigation systems and robotic assistance techniques have gained popularity to improve accuracy. Since the bone-mounted miniature SpineAssist (Mazor Robotics Ltd.) became the first U.S. Food and Drug Administration-approved robotic system for spine surgery in 2004, various robots have been developed and clinical trials using CT-based navigation systems have been conducted [
17]. However, according to a prospective randomized comparison to conventional freehand screw insertion, the accuracy assessment by Gertzbin-Robbins classification indicates that the conventional freehand technique (A+B, 93%) is superior to the robotic-guided technique (A+B, 85%) [
9]. A prospective randomized controlled trials (RCTs) performed by Kim et al. [
23] reported that there was no significant difference between robot-assisted techniques and conventional freehand techniques in terms of accuracy of the screw placement. Meanwhile, Fan et al. [
18] performed a meta-analysis based on 5 RCTs and several cohort studies and found that the robot-assisted technique showed significantly higher accuracy of pedicle screw placement than the freehand technique. Therefore, controversies regarding the accuracy and safety of robot-assisted pedicle screw placement remain. In our study, using the CUVIS-spine robot with a navigation system, we observed breached pedicles in 11.61% of screws in 113 consecutive patients, with the majority of breaches being less than 2 mm. Clinically acceptable screws, categorized as GRS grade A or B, accounted for 97.99% of our results, which is comparable or superior to previous studies using other robotic systems.
It is important to investigate quantitative accuracy data to improve surgical precision and minimize screw malposition in spine surgery [
4]. However, most of the previous studies demonstrated only GRS grade as the assessment of screw accuracy based on postoperative CT scans, and few studies have assessed quantitative measurements comparing preoperatively planned screw paths with the actual inserted screw paths. Godzik et al. [
4] reported mean 3D accuracy of 5.0±2.4 mm, mean 2D accuracy of 2.6±1.1 mm, and mean angular offset of 5.6°±4.3° among 17 patients (70 screws). In comparison, our study found lower errors in intraoperative 3D image-based planning, with mean entry offset of 2.86±1.64 mm, target offset of 1.99±2.13 mm, depth offset of 1.86±2.15 mm, and angular offset of 3.07±2.31 mm.
Furthermore, we performed a comparative analysis to evaluate the difference in accuracy between the open and percutaneous approaches for screw insertion. In the initial stages of clinical application of this new robotic system, there was a lack of confidence in its accuracy. Therefore, we performed the initial 5 cases using an open approach with a layer-by-layer verification to ensure precision. However, we discovered that this approach actually introduced additional sources of error rather than improving accuracy. The pushing effect exerted by the surrounding musculature caused greater errors than anticipated errors which could arise from bone surface skiving. Consequently, we shifted to using the percutaneous approach exclusively, which yielded more accurate results with reduced errors. The reason behind this improvement can be attributed to the percutaneous approach’s ability to evenly distribute resistance from the surrounding structures along the screw path, thereby minimizing errors. Based on our findings, we advocate for the principle of using percutaneous approach for the robot-assisted screw insertion in thoracolumbar area. In cases where an open approach is necessary, we emphasize the importance of maximizing muscle exposure to minimize resistance caused by the screwinserting instrument. Additionally, when using muscle retractors during open procedures, minimizing retractor movement after registration is crucial to avoid introducing errors.
We also conducted a comparative analysis to identify the difference in offset and GRS between the PLIF and OLIF groups. However, we would like to clarify that these 2 different interbody fusion methods were conducted exclusively at different institutions. Additionally, although there may be differences in interbody cage insertion methods, it is important to note that both groups underwent pedicle screw insertion using the same prone position with robot-assisted technique. According to our results, there were statistically significant differences between the PLIF and OLIF groups in entry offset, angular offset, and GRS. But it should be noted that these differences may be attributed to variations in the surgical institutions and surgeons involved, rather than solely the interbody fusion method. Therefore, careful interpretation of these results is warranted.
The robotic system we used in our study has several unique features that contributed to our relatively better results in terms of offsets. One notable feature is the real-time force navigation and patient displacement information provided by the system. The force navigation capability allows the system to detect the orientation and degree of lateral force from the surrounding tissue exerted on the surgical instrument in real time as it contacts the bone surface. This information helps the surgeon ensure that the instruments are being inserted along the planned path, thereby increasing the accuracy of screw placement. Additionally, the system can detect patient displacement, such as movement due to breathing, during the surgical procedure, which further enhances the accuracy of screw placement.
The real-time alarm system in the robotic system enables the surgeon to promptly respond to any force or displacement beyond acceptable levels. If such an event is detected, the surgeon can make adjustments, such as re-planning the screw path, adjusting the entry point or insertion angle, or enlarging the pilot hole using a larger end mill drill, to avoid errors. The surgeon’s expertise and experience play a crucial role in minimizing errors during the procedure, and the haptic feedback provided by the system during screw insertion is another important element in avoiding errors. This feedback is based on the surgeon’s perception and experience, which complements the robotic system’s capabilities. Overall, the combination of the robotic system’s real-time force navigation and patient displacement information, along with the surgeon’s decision-making and haptic feedback, contributes to improved accuracy in pedicle screw placement and helps minimize errors during the surgical procedure.
In terms of real-time feedback on lateral repulsive forces, the CUVIS-spine distinguishes itself from other spine robots. However, it is worth noting that some disadvantages of the CUVIS-spine relative to other robotic systems exist. Unlike other robotic systems, CUVIS-spine does not provide a dedicated tool using proprietary screws, which could enhance convenience. In other words, this system offers an open platform that allows for the use of various screw options, albeit without the dedicated tool, which may be considered a minor inconvenience.
One of the most significant advantages of robot-assisted spine surgery is the lower radiation exposure, particularly for the surgeon [
10,
13,
24,
25]. Previous studies have shown that spine surgeons can be exposed to radiation dose rates up to 12 times higher than other nonspinal musculoskeletal surgeons, which raises concerns about potential health risks associated with prolonged radiation exposure [
1,
3]. For instance, Roguin et al. [
26] reported cases of brain and neck tumors occurring in physicians who performed interventional procedures, with a disproportionate occurrence of tumors on the left side of the brain (85% of cases) due to the differential dose distribution of radiation exposure in interventionists who routinely worked with the left side of the head in closest proximity to the primary x-ray beam and scatter. Hyun et al. [
25] compared intraoperative radiation output recorded by C-arm mSv output and thermoluminescent dosimeters worn by surgeons between a robotic-guided minimally invasive spine surgery group and a fluoroscopic-guided open spine surgery group. The results showed that the C-arm mSv output was significantly lower in the robotic-guided group (0.13) compared to the fluoroscopic-guided group (0.27) (p=0.015), and the average per-screw radiation recorded by thermoluminescent dosimeters demonstrated a mean reduction of 62.5% in the use of radiation in the robotic-guided spine surgery group.
In our study, we used an intraoperative O-arm navigation system, except for only 6 cases where C-arm was used, which resulted in minimal radiation exposure for the surgeon and the operating room staff during the robotic-assisted spine surgery. This lower radiation exposure is a significant advantage of using robotic assistance in spine surgery, contributing to the safety and well-being of the surgical team.
Another challenge associated with robotic technology is the steep learning curve involved in mastering its use. Previous study by Urakov et al. [
27], which evaluated the feasibility of robot-assisted pedicle instrumentation involving residents and fellows in an academic environment, found no significant difference in the speed of pedicle instrumentation based on the operators’ years of experience or dedication to spine surgery. However, they noted a trend towards improved efficiency with more cases performed. Schatlo et al. [
28] reported that achieving satisfactory accuracy in robotic spine surgery may require around 25 cases per surgeon. In the first 10–20 cases, skilled supervision may be necessary to avoid complications during screw insertion, as inexperienced surgeons may face risks of inaccuracy.
Our results also showed a learning curve for robotic guidance, with a gradual decline in offsets and duration of the robot-assisted procedure over time. Based on the learning curves depicted in
Figs. 5 and
6, it is evident that there is little significant change in accuracy and surgical time after the initial 10–20 surgeries. This suggests that robotic-assisted surgery in this context offers the advantage of standardization. Once surgeons become familiar with the instruments used for screw insertion and adapt to the surgical process, we believe that achieving proficiency can be accomplished within approximately 10–20 surgeries.
However, there were several limitations in the analysis of the learning curve in this study. One limitation was the inability to include graphs for all 113 cases in
Figs. 5 and
6. This was attributed to the multicenter nature of the research, which involved surgeries conducted at different time points by different surgeons. As a result, we selected a subset of 65 cases that met the criteria for standardized surgical approaches, performed by the same surgeon, allowing us to assess the learning curve associated with robot-assisted spinal surgery. Future studies with larger sample sizes and diverse surgical settings are warranted to further validate our findings and generalize them to a broader population of patients undergoing robot-assisted spinal surgery. Secondly, the learning curve represented the general trend of decreasing surgical time as experience accumulates, but it did not provide a specific threshold or number of cases for achieving proficiency. To determine the necessary number of cases to achieve proficiency in robotic-assisted spine surgery, multiple factors need to be considered, including individual surgeon skill, complexity of cases, and the desired level of proficiency. It is essential to combine the findings from this study with other factors such as clinical judgment, additional performance metrics, and guidelines from professional societies to estimate the appropriate number of cases for achieving proficiency. Therefore, future studies exploring the learning curve and feasibility aspects of the robot procedure are required to investigate the differences among spine surgeons with varying levels of expertise, including residents, fellows, and professors.
Robotic assistance also offers the potential to lower the risk of infection, neurovascular damage, or revision surgery, as demonstrated in previous studies [
5,
8,
29-
31]. Kantelhardt et al. [
32] showed that only 1% of robotic procedures required revision surgery, compared to a reoperation rate of 12.2% for conventional techniques. The study also reported lower postoperative infection rates of 2.7% in robot-guided procedures compared to 10.7% in the conventional open surgery group. In our study, no patients required revision surgery, and there were no cases of neurovascular damage or infection. Late complications, such as pseudoarthrosis, screw loosening, screw pullout, screw breakage, or late spinal instability, will be evaluated with a long-term follow-up period in future studies.
The high cost of the instruments associated with spine robotic systems is a significant limitation that can reduce the possibility of wider use and development of this surgery. However, the application of robotic assistance in spine surgery can be cost-effective, resulting in decreased reoperation rates, decreased intraoperative and postoperative complication rates, and reduced length of hospital stays [
11]. Further investigations are warranted to thoroughly evaluate the cost effectiveness of robotic assistance in spine surgery.
There are several limitations to consider in this study. Firstly, the retrospective design of the study, rather than a RCT, is a notable limitation. Secondly, there is a lack of comparison with a group that underwent screw insertion using the conventional method. Instead, comparisons were made based on data from previous studies, focusing on numerical values. The absence of a direct comparison with a conventional method group limits our ability to assess the accuracy of the robot-guided system in relation to traditional techniques. Future research should consider including a control group that undergoes screw insertion using the conventional method to provide a more comprehensive evaluation of the robotic system’s accuracy. Thirdly, long-term follow-up was not included, which limits our understanding of potential complications that may arise in the delayed postoperative period. While no immediate neurovascular complications were observed in our study, it is important to assess the occurrence of complications over a longer duration. Finally, the experience of surgeons using the robot-guided system could potentially impact the outcomes. Although the multicenter design helps to mitigate this confounding factor to some extent, future studies should aim to include surgeons with varying degrees of experience. Prospective studies evaluating the efficacy of this robotic system in more complex cases, with long-term follow-up, are warranted.
In contrast to previous studies that mainly focused on clinical outcomes and gross descriptions such as GRS, one of the strengths of our study lies in its precise implementation of 3D offset calculations. Very few studies have conducted a thorough evaluation of 3D offset calculations with such accuracy, specifically in the context of the novel robotic technology. This makes our manuscript highly relevant and significant, as it contributes to the growing body of literature on the accuracy of robotic-assisted spinal surgery. By providing detailed insights into the 3D quantification of screw accuracy, our study addresses a critical aspect of this emerging field and emphasizes the importance of accuracy-based reports in evaluating the effectiveness of new robotic technologies.