 |
 |
- Search
|
|
||
Abstract
Objective
To investigate the value of Hounsfield units (HUs) as an independent predictor of failed percutaneous drainage of spinal tuberculosis paraspinal abscess under computed tomography (CT) guidance.
Methods
A retrospective analysis was conducted on 61 patients who underwent CT-guided percutaneous drainage for spinal tuberculosis paraspinal abscess between October 2017 and October 2020. Preoperative CT scans were used to measure the HUs of the abscess. Patients were categorized into successful drainage (n = 49) and failed drainage (n = 12) groups. Statistical analysis involved independent sample t-tests and chi-square tests to compare between the 2 groups. Binary logistic regression was performed to identify independent predictive factors for drainage failure. Receiver operating characteristic (ROC) curves were employed to ascertain risk factor thresholds and diagnostic performance.
Results
Among the patients, 49 experienced successful drainage while 12 faced drainage failure. The mean HUs of abscesses in the failed drainage group were significantly higher than those in the successful drainage group (p < 0.001). ROC analysis revealed an area under the curve of 0.897 (95% confidence interval, 0.808–0.986) for predicting drainage failure based on HUs. The optimal HU cutoff value for predicting drainage failure was 22.3, with a sensitivity of 91.7% and specificity of 69.4%.
Tuberculosis is a prevalent infectious disease worldwide, with an estimated one-third of the global population carrying the tuberculosis bacillus [1]. Despite a decline in its incidence, densely populated developing countries continue to experience significantly higher incidence and mortality rates compared to other infectious diseases, according to the tuberculosis surveillance report published by the World Health Organization [2]. Bone tuberculosis is a common form of extrapulmonary tuberculosis, with spinal tuberculosis accounting for over 50% of cases [3]. Studies indicate that 50% to 75% of spinal tuberculosis patients develop paraspinal tuberculosis abscess [4,5]. While anterior surgery was previously considered the standard treatment for patients with extensive abscess formation but no neurological impairment and mild bone destruction [6,7], it poses serious complications, such as nerve and vascular injury, abdominal visceral injury, or pneumothorax, due to the complex anatomical structure involved [8]. In recent years, percutaneous puncture drainage (PCD) combined with antituberculosis treatment has emerged as a minimally invasive alternative for treating tuberculosis psoas abscess [9,10]. However, PCD may fail due to reasons such as the pus being too thick or the drainage tube diameter being too small, leading to poor drainage [11,12], and ultimately requiring additional surgery to debride the abscess. This not only increases overall medical expenses but also increases the patient’s pain and nursing difficulty due to the retention of the drainage tube. Therefore, understanding the relationship between relevant factors and the efficacy of PCD is of great significance for guiding the selection of treatment methods for tuberculosis psoas abscess.
CT is the preferred imaging method for evaluating the extent of abscess, bone destruction, and other spinal tuberculosis-related changes [13]. It is considered the gold standard for diagnosing psoas abscess [14] and a routine examination for patients with spinal tuberculosis. Hounsfield units (HUs) are used to reflect the radiation density of tissues in CT images. The value of 0 is defined in water, -1,000 in air, and +1,000 in bones [15]. Considering previous animal experiments demonstrating a positive correlation between bile viscosity and density [16], this study employs HU as a measure of density to assess abscess viscosity. However, there is currently a lack of literature regarding the potential correlation between abscess viscosity and the efficacy of PCD treatment. As a result, we aimed to explore the degree of abscess viscosity using preoperative HUs as a risk factor for PCD failure.
The research protocol was approved by the ethnic committee of Southwest Hospital, Army Medical University ((B) KY2023032) and was performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
This study aimed to retrospectively investigate patients who underwent CT-guided puncture and drainage for paravertebral abscess from October 2017 to October 2020 in our hospital. Patients who met the inclusion criteria must have been diagnosed with spinal tuberculosis and accompanied by paravertebral abscess, received CT-guided puncture and drainage treatment, and had at least 24 months of follow-up, with regular CT follow-up before and after the operation and complete other follow-up data. Exclusion criteria included: (1) Diagnosis of other spinal infections other than spinal tuberculosis; (2) A history of anterior spinal surgery. Based on the inclusion and exclusion criteria, the patients who met the criteria were divided into 2 groups. Patients with poor puncture and drainage effect, large residual abscess requiring subsequent anterior tuberculosis lesion clearance surgery were defined as the puncture and drainage failure group. Patients with little or no residual abscess after puncture and drainage and successful removal of the puncture and drainage tube were defined as the puncture and drainage success group. According to the diagnostic and treatment algorithm for psoas abscess introduced by Yacoub et al. [17], we define cases where the remaining abscess diameter measured on CT or MRI > 3 cm as “large residual abscesses.”
The diagnosis of tuberculosis relies on clinical symptoms, laboratory tests (e.g., erythrocyte sedimentation rate [ESR] or C-reactive protein [CRP]), and radiological examinations (e.g., x-ray plain films, computed tomography [CT], and magnetic resonance imaging [MRI]). Confirmation is typically obtained through tissue culture, histological examination, or by demonstrating a positive response to antituberculosis chemotherapy [11,18,19]. All enrolled patients underwent a standardized preoperative antituberculosis treatment regimen, consisting of isoniazid, rifampicin, pyrazinamide, and ethambutol. During surgery, we routinely used a 12F pigtail drainage catheter for drainage, facilitating natural drainage through an external bag. Daily rinsing, consisting of 0.9% sodium chloride solution (20 mL) and isoniazid (200 mg), was adjusted in frequency based on purulent fluid characteristics 2 weeks postsurgery. Patients were advised to wear a brace for postoperative protection. Individualized antituberculosis treatment plans, guided by lesion drug sensitivity test results, typically continued for 18 months.
We retrospectively collected basic information on 2 groups of patients, including sex, age, body mass index (BMI), number of involved vertebral segments, duration of drainage tube placement, and HU of the preoperative abscess. The HU value of preoperative abscesses was determined by selecting a cross-section in the upper, middle, and lower regions of the abscess from the patient’s preoperative CT images, and using the elliptical region of interest tool in the image archiving and communication system to measure the abscess area as much as possible, avoiding areas of dead bone. The average of these 3 measurements was calculated as the HU value measurement for the respective patient’s abscess (Fig. 1). We used the American GE Optima 660 or the German Siemens SOMATOM Definition AS+CT scanner. The scanning parameters were as follows: tube voltage of 120 kV, tube current ranging from 30 to 70 mAs, slice thickness of 5 mm, and interlayer spacing of 5 mm. During the perioperative period, we evaluated the clinical efficacy of patients using the visual analogue scale (VAS) for back pain, the Oswestry Disability Index (ODI), CRP, and ESR indicators. It should be noted that for patients in the failed percutaneous drainage group, the postoperative follow-up data we analyzed were obtained after performing anterior approach open abscess debridement surgery.
For statistical analysis, we used IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation and analyzed using the independent sample t-test. Categorical variables were analyzed using the chi-square test or Fisher exact test. Within-group comparison was done using the paired t-test. To determine the cutoff value between the 2 groups, we used the receiver operating characteristic (ROC) curve, and the area under the ROC curve (AUC) was calculated for regional evaluation. We used binary logistic regression analysis to determine the independent predictors of PCD failure, and we expressed the results as odds ratio (OR) with a 95% confidence interval (CI). Statistical significance was set at p < 0.05 for differences.
The final analysis included 61 patients (41 males and 20 females) with a mean age of 38.3 ± 17.5 years, a mean BMI of 17.2 ± 1.1 kg/m2, and a mean follow-up time of 27.9 ± 3.0 months. According to the efficacy of the puncture and drainage, the patients were divided into the puncture and drainage failure group (n = 12) and the puncture and drainage success group (n = 49). According to the current follow-up results, no cases of tuberculosis recurrence were observed in all patients included in this study.
Table 1 shows the comparison of demographic and clinical data between the success group and the failure group. There were no significant differences between the 2 groups in terms of sex, age, BMI, number of affected vertebral segments, and follow-up time (p > 0.05). However, there were significant differences between the failure group and the success group in terms of the duration of indwelling drainage tube (50.0 ± 9.8 days vs. 33.7 ± 15.5 days, p = 0.001) and the HU value of the preoperative paravertebral abscess (29.5 ± 6.2 vs. 20.0 ± 4.3, p < 0.001).
The findings from the binary logistic regression analysis examining the factors predicting the need for surgical intervention due to PCD failure are presented in Table 2. The analysis revealed that a higher preoperative HU value of the paravertebral abscess was a significant independent predictor of the need for surgical intervention due to PCD failure (OR, 1.829; 95% CI, 1.084–3.087; p = 0.024). Additionally, the results of the ROC curve analysis (Table 3, Fig. 2) showed that the preoperative HU value of the paravertebral abscess had a strong predictive ability for PCD failure, with an AUC of 0.897 (95% CI, 0.808–0.986; p < 0.001). Based on our analysis, we identified a critical value of 22.3 for the preoperative HU value of the paravertebral abscess as a threshold for identifying patients with a higher risk of PCD failure, which had a high sensitivity (91.7%) and appropriate specificity (69.4%).
Table 4 and Fig. 3 show the evaluation of clinical outcomes for the 2 groups of patients. Preoperatively, there was no significant difference between the failed group and the successful group in VAS-back score (5.1 ± 1.0 vs. 5.4 ± 0.8) and ODI (55.8 ± 5.7 vs. 57.1 ± 5.6) (p > 0.05). However, on postoperative day 3, the VAS-back score of the failed group was significantly higher than that of the successful group (4.2 ± 0.8 vs. 3.4 ± 1.2, p < 0.05). There were no significant differences in VAS-back score and ODI between the 2 groups at the remaining postoperative follow-up time points. Preoperatively, there were no significant differences in CRP and ESR between the failed group and the successful group (67.9 ± 19.3 mg/L vs. 70.6 ± 14.5 mg/L and 70.2 ± 11.9 mm/hr vs. 66.0 ± 13.0 mm/hr, respectively, p > 0.05). However, the failed group had significantly higher levels of CRP (86.8 ± 26.1 mg/L vs. 59.8 ± 22.0 mg/L, p < 0.05) and ESR (77.7 ± 18.0 mm/hr vs. 53.7 ± 13.9 mm/hr, p < 0.001) on postoperative day 3, as well as significantly higher levels of CRP (40.4 ± 15.3 mg/L vs. 30.3 ± 5.4 mg/L, p < 0.001) on postoperative day 7, compared to the successful group. There were no significant differences in CRP and ESR between the 2 groups at the remaining postoperative follow-up time points. At the 3-month postoperative follow-up, all the efficacy evaluation indicators for the 2 groups of patients had returned to normal levels, with significant improvement compared to preoperative levels.
In thoracolumbar spinal tuberculosis patients with paraspinal abscess but no severe neurological damage or spinal deformity, the treatment approach should stress not only the significance of antituberculosis therapy but also the importance of minimally invasive debridement and abscess drainage [20,21]. In 1984, Mueller et al. [13] pioneered the use of PCD for treating iliopsoas muscle abscess. Since then, this technique has evolved and found broader applications in clinical practice. Numerous studies have confirmed that CT-guided percutaneous catheter drainage holds promise for effectively managing spinal tuberculosis and paraspinal abscess [22,23]. However, it’s worth noting that catheter drainage failure is not uncommon in clinical settings.
To effectively prevent failure of CT-guided PCD, it is essential to understand the associated risk factors. This study analyzed 61 patients with spinal tuberculosis and adjacent abscesses who underwent CT-guided PCD and found that the HU values of abscesses in 12 patients with failed PCD were significantly higher than those in 49 patients with successful PCD (29.5 ± 6.2 vs. 20.0 ± 4.3, p < 0.001), with higher HU values usually indicating more viscous abscesses. As per Poiseuille’s law, viscosity (η) and flow rate (Q) are inversely proportional-an increase in viscosity results in a decrease in flow rate [24]. This phenomenon occurs due to an increase in intermolecular interaction forces within the liquid, hindering its flow in the pipeline. Therefore, viscous abscesses are difficult to drain thoroughly, which was also confirmed in our cases. One patient with failed drainage had a viscous cheesy abscess during anterior approach surgery, and the HU value of the abscess measured by CT before the surgery was as high as 37.2 (see typical case in Fig. 4). In our clinical practice, we noticed that the form of abscess was fluid in some patients in acute phase, while for other patients in chronic phase or of delayed diagnosis, the lesions of spinal tuberculosis were viscous abscess, granulomatous inflammation with caseous necrosis. The viscous abscess, granulomatous tissue and caseous necrosis with higher HU value are difficult to be drained out through the catheter. By ROC curve analysis, the critical value of abscess HU was determined to be 22.3, which provided 91.7% sensitivity and 69.4% specificity. This suggests that for patients with a preoperative HU value greater than 22.3, CT-guided PCD should be performed more cautiously as there is a greater risk of PCD failure. This study also found that the duration of indwelling drainage catheters in the failed puncture group was significantly longer than that in the successful group (50.0 ± 9.8 days vs. 33.7 ± 15.5 days, p = 0.001), which may lead to more medical expenses and greater pain for patients. In conclusion, the results of this study show that there is a significant correlation between higher HU values of the adjacent abscess and the failure of PCD under CT guidance, and HU value can be an independent predictor of the failure of CT-guided PCD of spinal tuberculosis adjacent abscess. Therefore, spine surgeons should routinely evaluate the preoperative HU value of the adjacent abscess measured by CT scan when planning CT-guided PCD of spinal tuberculosis adjacent abscess. We cautiously recommend that in cases where the measured abscess HU value is less than 22.3, we consider that percutaneous abscess drainage has a higher success rate. In such circumstances, whether it is a simple paraspinal abscess or an anterior abscess that is difficult to clear with posterior surgery, we recommend considering percutaneous drainage guided by CT to treat the paraspinal abscess. Furthermore, the need for posterior internal fixation and fusion surgery can be determined based on the presence of vertebral instability or neurological impairment in the patient.
A small catheter, large abscess, or multiple compartments can hinder percutaneous drainage success. In the study of Pieri et al. [25], an 8F catheter was commonly used. When results were unsatisfactory, they shifted to a 10F catheter, achieving a 100% success rate. Increasing catheter diameter, a conventional solution for poor drainage, may raise organ damage risks and costs. In our study, we employed a bold approach, using a 12F catheter to minimize narrowing-related drainage issues, albeit potentially causing more local discomfort. One study suggests that catheter size exceeding 8.3F does not significantly impact outcomes [26]. Yacoub et al. [17] recommend percutaneous drainage for abscesses > 3 cm, while smaller ones respond to standard antituberculosis treatment. In spinal tuberculosis, the influence of abscess size and compartments on drainage efficacy is underreported. Yet, liver abscess studies offer insights due to similarities. Larger (> 5 cm) abscesses are prone to drainage failure due to their substantial purulent content [27]. Septations partitioning abscesses complicate drainage, permitting access to only one chamber. Gupta et al. [11] note this in paraspinal abscesses, echoing liver abscess findings [27,28]. Our study of 12 failed drainage cases found no significant septations. We emphasize CT-guided catheter placement with repeat scans for monitoring and, if septations are detected, additional catheter placement is considered. Some propose using guidewires to disrupt septations, though it lacks scientific validation. The research findings regarding the impact of multichambered abscesses on the effectiveness of percutaneous drainage reveal that certain multiloculated abscesses can be drained successfully with a single catheter, as septations observed on imaging may not completely isolate the compartments, allowing communication between them. The final conclusion is that percutaneous drainage is a safe and effective procedure in the treatment of pyogenic liver abscess, regardless of abscess complexity and/or multiplicity [29].
In this study, patients undergoing CT-guided percutaneous abscess drainage achieved similar long-term clinical outcomes as those undergoing open abscess removal surgery. Specifically, there were no significant differences in CRP, ESR, VAS, and ODI indicators between the 2 groups of patients at the last follow-up after surgery. This is consistent with the conclusions reached in other studies on CT-guided percutaneous drainage for the treatment of spinal tuberculosis adjacent to abscesses [22,23]. In addition, combining local chemotherapy can significantly increase drug concentration [30], thereby improving efficacy while reducing drug resistance and side effects [9,31]. The emergence of resistance to first-line antituberculosis drugs has become a growing global challenge and is a significant factor in tuberculosis recurrence [32]. Preserved purulent fluid can be employed for polymerase chain reaction, bacterial culture, and drug susceptibility testing, aiding in tuberculosis diagnosis and treatment. There were significant differences in CRP, ESR, and VAS indicators at 3 days postsurgery, as well as CRP indicators at 7 days postsurgery, between the 2 groups of patients. It is not difficult to see that in the early postoperative period, patients undergoing CT-guided percutaneous abscess drainage showed significant advantages over patients undergoing anterior open surgery, which may be due to iatrogenic trauma. Firstly, larger surgical incisions and excessive tissue stripping inevitably cause significant pain around the surgical site after surgery. Secondly, larger wounds exacerbate the body’s inflammatory response, leading to a significant increase in inflammatory indicators such as CRP and ESR. Although the effect of CT-guided percutaneous drainage is influenced by many factors, considering its clinical efficacy, small trauma, short operation time, and low incidence of complications in appropriate indications, CT-guided abscess puncture is still one of the better choices for the treatment of spinal tuberculosis adjacent to abscesses. We present a successful cure of spinal tuberculosis with an accompanying abscess using CT-guided percutaneous drainage (Fig. 5). The patient, a young female, had preoperative imaging revealing a paraspinal abscess in the anterior region of T6–T10. Although there was mild vertebral body destruction, clinical symptoms and physical examination did not indicate notable spinal instability or neurological damage. Consequently, the patient underwent CT-guided percutaneous drainage, followed by postoperative natural drainage using a drainage bag. This treatment was combined with both systemic and local chemotherapy. Ultimately, the patient achieved complete recovery.
CT is a commonly used diagnostic method for spinal tuberculosis patients, as it does not increase additional medical expenses or radiation exposure. HU values are an effective measure of radiation density in CT images and have been shown to be superior to dual-energy x-ray absorptiometry in reflecting local bone density [33], while also partially reflecting the viscosity of fluids. We used the average HU value of the preoperative paraspinal abscess to evaluate the viscosity of the abscess. This method offers several advantages. Firstly, it is simple and fast, requiring only the use of the area of interest tool in the PACS (picture archiving and communication system) system, without the need for special equipment or software support. Secondly, by selecting specific areas for measurement, it can reduce the influence of factors such as bone destruction, degenerative hypertrophy of the psoas muscle and vertebral body on the measurement results, ensuring measurement accuracy.
During the follow-up period, none of the patients in our study experienced recurrence, which can be attributed to our rigorous adherence to individualized and comprehensive antituberculosis treatment based on drug susceptibility results from lesion tissue analysis. Furthermore, we closely monitored patients postoperatively and provided regular outpatient follow-ups to ensure proper medication usage. It is important to note that without antituberculosis treatment, regardless of the chosen approach, treatment failure is inevitable. Previous research has indicated that recurrence events are mostly unrelated to the surgical method employed but rather linked to inadequate antituberculosis drug therapy [9]. In our study, all patients underwent a standardized quadruple antituberculosis chemotherapy regimen before surgery. Subsequently, they received a minimum of 18 months of postoperative treatment tailored to their drug sensitivity results. We strongly emphasize the significance of maintaining an adequate duration of chemotherapy to prevent recurrence and the emergence of drug-resistant strains. This study has some limitations that need to be acknowledged. Firstly, it is a retrospective study, and therefore, the potential for subjective selection bias in patient selection cannot be ruled out. Secondly, the sample size is relatively small, and the follow-up period is short. In addition, the study did not examine the relationship between the diameter of the drainage tube, the size of the abscess, and the efficacy of the drainage treatment. Finally, future large-scale, prospective studies are needed to validate the findings of this study.
The study reveals that the average HU value of paravertebral abscesses prior to percutaneous drainage under CT guidance was significantly higher in patients who required abscess removal due to failed drainage compared to those who had successful drainage. The HU value before the procedure can be an independent predictor of the failure of CT-guided percutaneous drainage of spinal abscesses. Therefore, measuring the HU value of the abscess in the pre-procedural CT scan can serve as a quick, relative reliable, and straightforward method for physicians to develop treatment plans.
NOTES
Fig. 1.
This figure demonstrates our study’s method for determining Hounsfield unit (HU) values in computed tomography using the elliptical region of interest tool. The left image depicts the selection of planes at the upper, middle, and lower sections of the abscess coronal plane for measuring abscess HU values. The right image displays the HU values generated by the imaging software program.
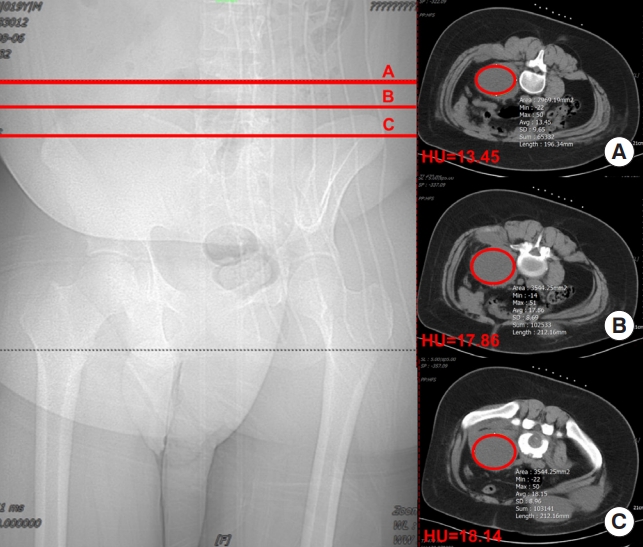
Fig. 2.
Receiver operating characteristic curve for the Hounsfield unit value as a predictor of puncture drainage failure, with an area under the curve of 0.897, sensitivity of 91.7% and specificity of 69.4%. AUC, area under the curve.
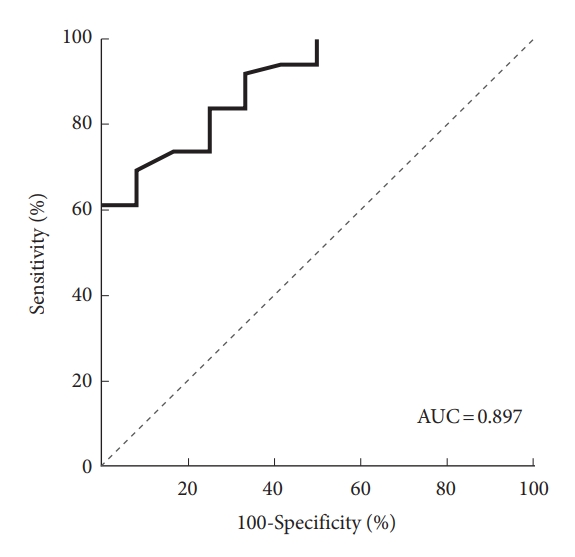
Fig. 3.
Graph of visual analogue scale (VAS; A), Oswestry Disability Index (ODI; B), C-reactive protein (CRP; C), and erythrocyte sedimentation rate (ESR; D) perioperative and in follow-up. SD-group, successful drainage group; FD-group, failed drainage
group; preop, preoperative; postop, postoperative. *p < 0.05. ***p = 0.001. ***p < 0.001.
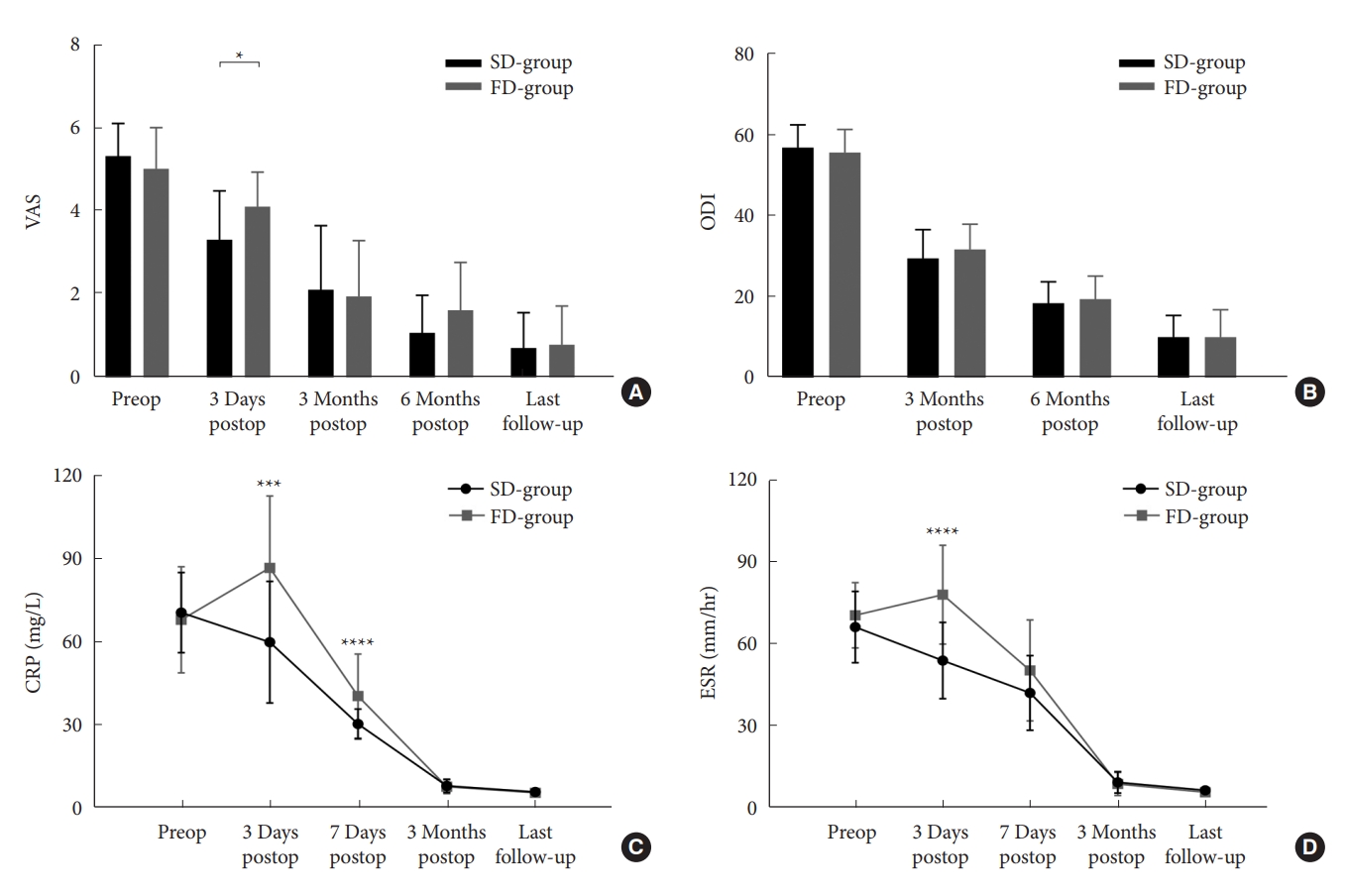
Fig. 4.
Typical case: perioperative radiographic examination and intraoperative images of a young female patient with spinal tuberculosis. Panels A and B demonstrate preoperative anteroposterior and lateral x-rays of the lumbar spine, Panel C shows preoperative computed tomography (CT) images of the lumbar spine, while panels D and E display preoperative magnetic resonance imaging (MRI) images of the lumbar spine (preoperative evaluation revealed severe vertebral destruction at the L3/4 level with the formation of a paravertebral abscess). Panels F and G depict postoperative anteroposterior and lateral x-rays of the lumbar spine (the patient underwent posterior approach spinal tuberculosis lesion debridement, bone graft fusion, and internal fixation; due to incomplete clearance of the psoas abscess anteriorly during surgery, CT-guided abscess drainage was performed). Panel H illustrates postoperative MRI images of the lumbar spine (the effect of abscess drainage was unsatisfactory, residual abscess was still present). Panel I demonstrate the most recent follow-up lumbar spine CT images (showing continuous bridging bone formation within the intervertebral space, without apparent psoas abscess). Panel J presents intraoperative photographs of anterior approach abscess debridement (the abscess is visible as a thick, cheesy material).

Fig. 5.
This is a case of a young female patient with thoracic spine tuberculosis who achieved a cure solely through computed tomography (CT)-guided percutaneous drainage treatment. Panels A and B display preoperative thoracic spine x-ray images in the anteroposterior and lateral views, Panels C and D show preoperative thoracic spine CT images, and Panel E presents preoperative thoracic spine magnetic resonance imaging (MRI) images (preoperative assessment indicated the presence of an anterior abscess from T6 to T10, with relatively mild vertebral bone destruction and no evident vertebral instability or neurological impairment). Panel F represents an immediate postoperative x-ray image (the patient underwent CT-guided percutaneous drainage for paraspinal abscess). Panel G shows a postoperative thoracic spine MRI image (taken 2 months after percutaneous drainage treatment, with the drainage catheter already removed, and no evident anterior abscess). Panels H–J depict recent follow-up thoracic spine x-ray and CT images (indicating the absence of recurrent spinal tuberculosis, abscess formation, and progressive bone destruction).
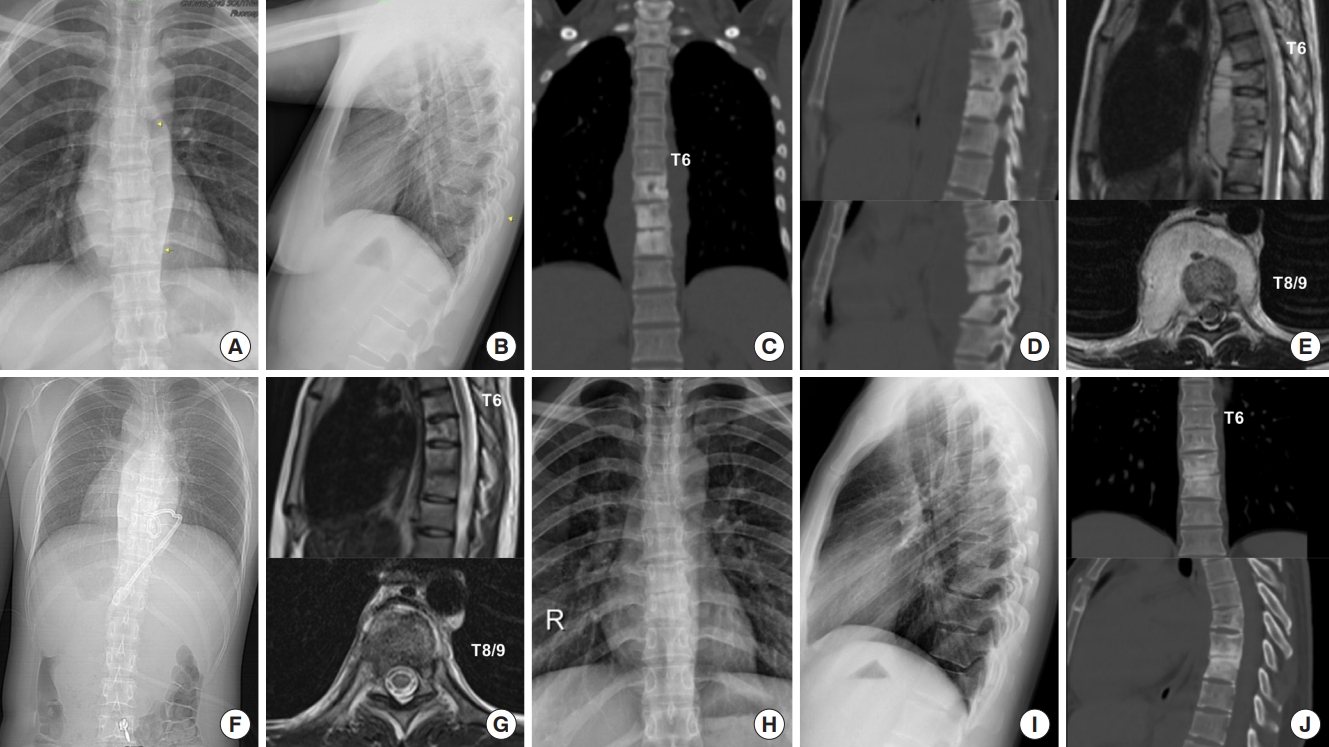
Table 1.
Demographic and clinical data
Table 2.
Logistic regression analysis assessing future surgical intervention for puncture drainage failure
Table 3.
Results of receiver operating characteristic analysis
| AUC (95% CI) | p-value | Cutoff | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| Hounsfield units | 0.897 (0.808–0.986) | < 0.001 | 22.3 | 91.7% | 69.4% |
Table 4.
Clinical efficacy evaluation
REFERENCES
1. Varatharajah S, Charles YP, Buy X, et al. Update on the surgical management of Pott's disease. Orthop Traumatol Surg Res 2014;100:229-35.

2. World Health Organization. Global tuberculosis control: WHO Report 2011. Geneva (Switzerland): World Health Organization; 2012.
3. Jin W, Wang Q, Wang Z, et al. Complete debridement for treatment of thoracolumbar spinal tuberculosis: a clinical curative effect observation. Spine J 2014;14:964-70.


4. Lindahl S, Nyman RS, Brismar J, et al. Imaging of tuberculosis. IV. Spinal manifestations in 63 patients. Acta Radiol 1996;37:506-11.


5. Rajasekaran S, Shanmugasundaram TK, Prabhakar R, et al. Tuberculous lesions of the lumbosacral region. A 15-year follow-up of patients treated by ambulant chemotherapy. Spine (Phila Pa 1976) 1998;23:1163-7.

6. Lü G, Wang B, Li J, et al. Anterior debridement and reconstruction via thoracoscopy-assisted mini-open approach for the treatment of thoracic spinal tuberculosis: minimum 5-year follow-up. Eur Spine J 2012;21:463-9.




7. Li M, Du J, Meng H, et al. One-stage surgical management for thoracic tuberculosis by anterior debridement, decompression and autogenous rib grafts, and instrumentation. Spine J 2011;11:726-33.


8. Mobbs RJ, Phan K, Daly D, et al. Approach-related complications of anterior lumbar interbody fusion: results of a combined spine and vascular surgical team. Global Spine J 2016;6:147-54.




9. Dinç H, Ahmetoğlu A, Baykal S, et al. Image-guided percutaneous drainage of tuberculous iliopsoas and spondylodiskitic abscesses: midterm results. Radiology 2002;225:353-8.


10. Hou X, Sun X, Zhang Z, et al. Computed tomography-guided percutaneous focal catheter infusion in the treatment of spinal tuberculosis. Acta Orthop Belg 2014;80:501-7.

11. Gupta S, Suri S, Gulati M, et al. Ilio-psoas abscesses: percutaneous drainage under image guidance. Clin Radiol 1997;52:704-7.


12. Rotman JA, Getrajdman GI, Maybody M, et al. Effect of abdominopelvic abscess drain size on drainage time and probability of occlusion. Am J Surg 2017;213:718-22.



13. Mueller PR, Ferrucci JT Jr, Wittenberg J, et al. Iliopsoas abscess: treatment by CT-guided percutaneous catheter drainage. AJR Am J Roentgenol 1984;142:359-62.


14. Santaella RO, Fishman EK, Lipsett PA. Primary vs secondary iliopsoas abscess. Presentation, microbiology, and treatment. Arch Surg 1995;130:1309-13.


15. Sudhyadhom A. On the molecular relationship between Hounsfield unit (HU), mass density, and electron density in computed tomography (CT). PLoS One 2020;15:e0244861.



17. Yacoub WN, Sohn HJ, Chan S, et al. Psoas abscess rarely requires surgical intervention. Am J Surg 2008;196:223-7.


18. Sharif HS. Role of MR imaging in the management of spinal infections. AJR Am J Roentgenol 1992;158:1333-45.


19. Krishnan A, Patkar D, Patankar T, et al. Craniovertebral junction tuberculosis: a review of 29 cases. J Comput Assist Tomogr 2001;25:171-6.


20. Zhang X, Zhang Z, Zhang Y, et al. Minimally invasive retroperitoneoscopic surgery for psoas abscess with thoracolumbar tuberculosis. Surg Endosc 2015;29:2451-5.



21. Büyükbebeci O, Seçkiner I, Karslı B, et al. Retroperitoneoscopic drainage of complicated psoas abscesses in patients with tuberculous lumbar spondylitis. Eur Spine J 2012;21:470-3.




22. Zhang Z, Hao Y, Wang X, et al. Minimally invasive surgery for paravertebral or psoas abscess with spinal tuberculosis - a long-term retrospective study of 106 cases. BMC Musculoskelet Disord 2020;21:353.




23. Cantasdemir M, Kara B, Cebi D, et al. Computed tomography-guided percutaneous catheter drainage of primary and secondary iliopsoas abscesses. Clin Radiol 2003;58:811-5.


25. Pieri S, Agresti P, Altieri AM, et al. Percutaneous management of complications of tuberculous spondylodiscitis: short-to medium-term results. Radiol Med 2009;114:984-95.



26. Gobien RP, Stanley JH, Schabel SI, et al. The effect of drainage tube size on adequacy of percutaneous abscess drainage. Cardiovasc Intervent Radiol 1985;8:100-2.



27. Tan YM, Chung AY, Chow PK, et al. An appraisal of surgical and percutaneous drainage for pyogenic liver abscesses larger than 5 cm. Ann Surg 2005;241:485-90.



28. Yu SC, Ho SS, Lau WY, et al. Treatment of pyogenic liver abscess: prospective randomized comparison of catheter drainage and needle aspiration. Hepatology 2004;39:932-8.


29. Liu CH, Gervais DA, Hahn PF, et al. Percutaneous hepatic abscess drainage: do multiple abscesses or multiloculated abscesses preclude drainage or affect outcome. J Vasc Interv Radiol 2009;20:1059-65.


30. Nene A, Bhojraj S. Results of nonsurgical treatment of thoracic spinal tuberculosis in adults. Spine J 2005;5:79-84.


31. Yin XH, Zhang HQ, Hu XK, et al. Treatment of pediatric spinal tuberculosis abscess with percutaneous drainage and low-dose local antituberculous therapy: a preliminary report. Childs Nerv Syst 2015;31:1149-55.




- TOOLS
- Related articles in NS
-
Journal Impact Factor 3.2



























