 |
 |
- Search
|
|
||
Abstract
Objective
The application of the da Vinci Surgical System in neurosurgery is limited due to technical difficulties requiring precise maneuvers and small instruments. This study details the advantages and disadvantages of robotics in neurosurgery and the reachable range of the transoral approach to lesions of the skull base and upper cervical spine.
Methods
In a cadaver study, the da Vinci Xi robot, lacking haptic feedback, was utilized for sagittal and coronal approaches on 5 heads, facilitating dura suturing in 3, with a 30°-angled drill for bone removal.
Results
Perfect exposure of all the nasopharyngeal sites, clivus, sellar, and choana, including the bilateral eustachian tubes, was achieved without any external incisions using this palatal split approach of transoral robotic surgery. The time required to perform a single stitch, knot, and complete single suture in robotic suturing of deep-seated were significantly less compared to manual suturing via the endonasal approach.
Conclusion
This is the first report to show the feasibility of suturing the dural defect in deep-seated lesions transorally and revealed that the limit of reach in the coronal plane via a transoral approach with incision of the soft palate is the foramen ovale. This preclinical investigation also showed that the transoral robotic approach is feasible for lesions extending from the sellar to the C2 in the sagittal plane. Refinement of robotic instruments for specific anatomic sites and future neurosurgical studies are needed to further demonstrate the feasibility and effectiveness of this system in treating benign and malignant skull base lesions.
Robot-assisted surgery has been adopted in various surgical fields and is also expected to be adopted in the neurosurgical field. This robot-assisted technology is used to perform safer and more reliable minimally invasive surgery. However, existing robots are not equipped with the drills that are required in the neurosurgical field.
Robotic neurosurgery can be divided into 3 categories. The first is leader-follower systems, such as the da Vinci Xi Surgical System, which allow for deep surgery using endoscopes and manipulation in areas that are difficult to reach with tools. The second category relates to assisting surgery to improve the performance and efficiency of stereotactic and spine screw insertion surgeries. The third category regards processes, systems, or tools that complement existing surgical procedures.
In neurosurgery, there has been a gradual shift from microscopy to endoscopy. For example, in lesions at the skull base, including the pituitary gland, the Hardy approach using a microscope was first employed; endoscopic surgery is now mainstream. Robot-assisted surgeries should be considered in the future.
Several studies and clinical reports have used the da Vinci Xi Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA, USA) in the transoral approach [1-4], in transoral odontoidectomy [5-7], and in the transmaxillary [8,9], transnasal [10], supraorbital [11], transorbital [12], and transcervical approaches [13], but few studies have reported using da Vinci Xi Surgical System to verify reachability via a transoral approach.
This study detailed suturing techniques in deep-seated lesions, the reachable range of the transoral approach to lesions of the skull base and upper cervical spine, as well as the advantages and disadvantages of robotics in neurosurgery.
A da Vinci Xi robot was used in a cadaver laboratory. Five cadaver heads without any congenital abnormalities were used in this study; 3 were used for the sagittal approach and 2 for the coronal approach. Suturing of the dura was performed in 3 cadavers. All procedures were performed using the da Vinci Xi robot, which does not send haptic sensations to the operating surgeon. The robotic arms of the da Vinci Xi consisted of 8-mm diameter Black diamonds, Potts scissors, and Dubakey and monopolar forceps. An angled drill (Maedas rex, Medtronic, Minneapolis, MN, USA) was used for bone removal transorally.
All procedures involving human participants were conducted in accordance with the ethical standards of the Institutional Research Committee of Fujita Health University (HM20-134). This study was performed per the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all the patients.
The approach to the skull base region using the oral method with the da Vinci Xi Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA, USA) was validated using a cadaver. The patient was placed supine with minimal cervical extension using a shoulder pillow due to the upward entry path (Fig. 1A), and the da Vinci was introduced from the patient’s left side (Fig. 1B). The surgeon faced the console while performing the surgery (Fig. 1C). In actual surgery, the surgeon is not in a clean field. The assistant performed various tasks in the clean field, such as aspirating smoke and fluid from the monopole in the operative field, informing the surgeon of arm interference, adjusting the arm and camera, and changing the arm. In the transoral method, only 2 arms can fit because of the limited size and orientation of the entry port. The arms included a monopolar and a Dubakey forceps as well as an 8-mm Potts scissor, and each was used with 1 hand. The camera used for observation was 8 mm in diameter and faced 30° upward (Fig. 1D).
An FK-WO aperture (Olympus, Tokyo, Japan) was used to develop the operative field (Fig. 2A). The soft palate was incised laterally by a monopolar and retracted to achieve the maximum field of view (FOV). The nasopharynx and posterior nares were exposed. Eustachian tubes were opened on both sides (Fig. 2B). The surgical view from the nasopharynx to the middle pharynx was observed; the mucosal muscles with pedicles on the caudal side of the posterior wall of the pharynx were detached from the clivus in one piece with a monopolar, and a flap was created (Fig. 2C). When the muscles and mucosa were inverted, the bony structures of the clivus, craniocervical junction area, foramen magnum, and the anterior arch of the C1 vertebra and odontoid process of the C2 vertebra were exposed (Fig. 2D). From the endonasal endoscopic view, the nasopharynx and mucosal flap could be seen from different angles (Fig. 2E). After the mucosa of the posterior nostril was dissected to identify the nasal septal bone, the clivus and inferior wall of the sphenoid sinus were removed by hand using an angled drill (Fig. 2F). When the lower wall of the sphenoid sinus was widely opened, the base of the sellar floor was observed in front, followed by the posterior nasal aperture and clivus. Subsequently, the base of the sellar floor was removed using an angled drill, and the bone window was opened (Fig. 2G). The dura was incised, and the pituitary gland was identified, demonstrating that the da Vinci arm was sufficient for surgical manipulation of the sellar lesion (Fig. 2H). This confirmed that it was possible to reach and manipulate a wide area from the base of the sellar and pituitary gland to the C2 vertebra in the sagittal plane (Fig. 2I). We observed that extensive and sufficient removal of the bone allows easy manipulation of the dura mater. However, while the usual nasal endoscopic approach involves entering from the anterior side, removing the anterior wall of the sphenoid sinus, and moving toward the sellar floor, this approach requires familiarity with the different directions of entry, that is, removing the inferior wall of the sphenoid sinus and moving downward and upward to the sellar floor.
Subsequently, the da Vinci Xi Surgical System was advanced in the lateral direction. Due to the soft tissue on the lateral side, no drilling was necessary, and dissection was performed using Dubakey forceps and a monopolar. The soft palate was incised to achieve the FOV (Fig. 3A). The palatopharyngeal and palatoglossus muscles were dissected from soft tissue (Fig. 3B). The most important structures were the internal carotid artery (ICA) and internal jugular vein, which were firmly visualized. During the surgery, doppler and indocyanine green fluorescence angiography were used to confirm these structures (Fig. 3C). The ICA enters the cranial space through the foramen lacerum. Following the ICA toward the skull base, the foramen lacerum was identified, and the bottom surface of the skull base was reached (Fig. 3D). The muscles and connective tissues were dissected to expose the middle fossa. The lateral limitation of this reach by the da Vinci Xi was identified as the foramen ovale (Fig. 3E). The foramen spinosum could be identified; however, the arm can be difficult to operate around the foramen spinosum (Fig. 3F).
We examined whether the da Vinci Xi was capable of suturing deep-seated lesions. We removed the clivus bone to the edge of the eustachian tube to obtain a maximal working space of 2 cm in width. The dura of the clivus was exposed. A 1-cm square defect was created in the dura mater of the clivus (Fig. 4A). The fascia was harvested from the thigh for approximately 15 mm on each side. It was marked with a cross in blue ink to facilitate positioning and suturing (Fig. 4B). First, the needle was grasped parallel to the forceps and the midline of the upper side was sutured using 7-0 proline. The da Vinci Xi arms used 8-mm diameter Dubakey forceps in both hands. All da Vinci arms were 8 mm in diameter and had 7 degrees of freedom. The most delicate forceps are the black diamond, but the power of the needle grasp is weak, and the stiffness of the fascia often causes the needle to rotate. Using the blue mark as a landmark, the needle was passed through the fascia and into the dura, as shown in Fig. 4C, to suture the fascia over the inlay (Fig. 4C). Ligation was performed using surgical sutures with the tip of robotic forceps (Fig. 4D). Because the bone window was only 2 cm wide, the thread had to be pulled back and forth, not horizontally, during tightening (Fig. 4E). Thread was then applied to the right side of the defect. The needle was grasped at a right angle relative to the forceps. Similarly, the fascia was threaded first, followed by the dura mater and the fascia was overlapped in a inlay manner. Ligation was performed in the same manner (Fig. 4F). Although the da Vinci has no haptics and relies on visual information for the strength of thread tightening, it can be pulled without cutting the thread by pulling too hard. Thus, visual information alone may be sufficient. Subsequently, a thread was applied to the bottom edge. As before, the thread was applied to the fascia and dura, as if overlapping in an inlay manner. The needle was grasped downward, parallel to the forceps (Fig. 4G). On the left side, the needle was grasped at a right angle to the forceps with the surgeon’s left hand, even though the surgeon was right-handed (Fig. 4H). The needle was easily threaded by the nondominant hand. Eight additional sutures were performed circumferentially, tightly suturing the fascia to the dura mater (Fig. 4I). The order of suturing is indicated by the numbers in Fig. 4I. We confirmed that deep suturing was feasible using the da Vinci Xi. The time taken for one stitch with the needle, knotting, and one complete suture were measured for both the da Vinci Xi and the nonrobotic suturing technique in endoscopic endonasal surgery (Table 1).
Advantages of the surgical robot include the following: (1) Fine, precise, and easy manipulation in difficult-to-access areas: robots enable sophisticated manipulation in areas that are difficult for the human hand to reach. These characteristics preserve function, reduce complications, decrease blood loss, minimize invasive procedures comparable with common endoscopic procedures, and reduce postoperative pain. (2) A high-magnification FOV with 3-dimensional (3D) high definition: the surgical robot displays a high-resolution 3D high-definition FOV and can be operated while maintaining distance. Magnification is adjustable, with “x1,” “x2,” and “x4” magnification options, and the camera can get as close as 2 cm from the object for more detailed observation. However, care should be taken to avoid interference from these tools. (3) Tremor filtration and instrument position memory: the tremor filtration provided by many surgical robots facilitate fine manipulation. In addition, clutch operation allows movement beyond the range of motion of the human hand. For example, the da Vinci’s arm has 7 degrees of freedom. Furthermore, the motion scale is adjustable, and the ratio of hand-to-arm motion can be changed to “1:1.5,” “1:2,” or “1:3” to achieve more stable and fine manipulation.
Disadvantages of the surgical robot include the following: (1) Generally speaking, the lack of haptic information during robotic surgery may increase the risk of unintended tissue damage and tool interference because it relies solely on visual information. Because of the risk of interference between the robotic arms and jumping, adding a function to warn against arm-to-arm contact is essential. Initially, the haptic feedback was considered necessary; however, the present verification showed that issues did not occur due to the lack of haptic feedback. Our technique relied more on visual information than on tactile feedback. Consequently, the absence of haptic feedback in the da Vinci system was not as problematic as expected. (2) Many robotic surgical systems are not equipped with a drill function; however, the drill is a necessary tool in the neurosurgical field. (3) Although there is no delay in digital imaging, the pixels in the digital image may become coarser at higher magnifications. A higher magnification of the images is required for neurosurgical areas. (4) The inconvenience of suction: In neurosurgery, where cerebrospinal fluid floods the surgical area, suction is very important, and suction is usually held in one hand for microscopic and endoscopic surgeries. Existing suction is inadequate for neurosurgery. (5) Because robots with improved surgical accuracy depend on navigation, navigation accuracy is important. Brain shift, such as cerebrospinal fluid loss and gravity-induced brain deformation, is a challenge. (6) There is a balance between cost and performance: the implementation of robotic surgical systems is expensive, and the balance between performance and cost is an issue. There are reports that show robotic surgery costs a hospital 1.4 to 2 times as much as identical nonrobotic procedures such as abdominal surgery [14] and hysterectomy [15]. Another consideration is the relatively long time required for setup due to the large size and weight of the equipment. We summarized the advantages and disadvantages in Table 2.
Robotics has been introduced in neurosurgery with 3 main objectives: (1) to improve surgical techniques, (2) to improve surgical accuracy, and (3) to provide surgical assistance. In addition, the robots used for each of these purposes are different. First, leader-follower type robots contribute to improving surgical techniques; Da Vinci (Intuitive Surgical, Inc.) and Hinotori (Medicaroid Inc., San Jose, CA, USA), which are widely used in clinical practice, are equivalent and in use in Japan. Second, to improve surgical accuracy, stereotactic robotic systems exist to contribute to improving surgical accuracy; the Neuromate (Renishaw plc, Wotton-under-Edge, UK) was introduced in Japan in 2000; however, interest waned for a time due to limited indications. Recently, Rosa one (Zimmer Biomet, Warsaw, IN, USA), Stealth Autoguide (Medtronic), and Cirq (Brainlab, Munich, Germany) have been introduced to the market. Previously, Neuromate [16], Neuroptic T-30 [17], and a prototype by Chumnanvej et al. [18] have been reported. The main focus has been on deep electrode insertion and stereotactic biopsy, such as deep brain stimulation and stereoelectroencephalography (SEEG). SEEG reportedly reduces operative time compared with surgery using a conventional frame [19]. In addition, there are devices, such as Mazor X (Medtronic) and ExcelsiusGPS (Globus Medical, Audubon, PA, USA), that use a stereotactic approach for spinal fusion and provide navigation functions combined with intraoperative computed tomography imaging using an O-arm [20]. However, their introduction has not progressed in Japan because they are not covered by insurance, and the additional fee for robotic surgery does not apply. Third, to provide surgical assistance, a less common surgical assistance system used in other fields is the surgical support robot developed by the team of Shinshu University. The “EXPERT” was first presented in 2009 [21], and underwent subsequent improvements; in 2018, the “iArms” was jointly developed by Denso and Tokyo Women’s Medical University. Previously the Evolution 1 [22], Stewart Platform [23], Bresciea endoscope assistant robotic holder [24], Medineering [25], and ENDOFIX exo [26] were reported. These surgical robots feature a “hand-placement” function that follows the surgeon’s hand movements during surgery using a passive mechanism. A “hand rest” function reduces the surgeon’s hand tremors and fatigue and improves operability. This technology is expected to be a third-hand technique that supports the surgeon and may be useful when performing surgery.
In a literature review, Pangal et al. [27] reviewed 22 articles on robotic skull base neurosurgery, as well as flexible endoscopy using The Flex system [28], Versius [29]. Carrau et al. [1] and Chauvet et al. [30] have reported clinically on transoral skull base surgery. Additionally, the transoral reachable limit in the sagittal section has been reported as reaching from the sellar floor to the odontoid during odontoidectomy [7,31]. These findings align with the results of our own study. However, in the coronal section, a lateral limitation has not yet been determined. Pathologies in the infratemporal fossa and parapharyngeal fossa are rare and usually treated by otolaryngology, not neurosurgery. Currently, lesions in the middle and inferior pharynx are commonly treated by the da Vinci robot via a transoral approach [32]. Kupferman et al. [8] reported that it was possible to suture in deep-seated lesions, especially the anterior cranial fossa, using robotic arms, in which the arms were introduced by a bilateral transmaxillary transantral approach to the nasal cavity. This was the first reported approach of 8-mm robotic arms through the nasal cavity. However, most tumor resection procedures are performed through the endonasal route. In the case of dural reconstruction, a bilateral transmaxillary transantral approach should be made and a robot can be introduced. Currently, a nasoseptal flap is commonly used for the closure of dural defect, and in most cases, liquorrhea can be treated successfully. Previous authors have written that the major limitations were clinical applicability, suture materials, and biological materials, and did not describe the procedures in detail, such as kinds of thread, how to make knots, or problems with this method. Our transoral route is advantageous in providing quick access to the lesion and a wider space than maxillary sinus and antrum and nasal cavity approaches used in previous reports.
Compared with other surgical fields, neurosurgery has unique limitations and challenges in applying robotic surgery. In other areas, it is possible to insert a robotic arm from multiple directions; however, in the neurosurgery the procedures are performed in a narrow and deep space in the only one direction, and improvements are needed for this purpose.
The arm of the da Vinci Xi and the camera are 8 mm in diameter. The latest model, the da Vinci Sp, is said to have an extended arm with a diameter of approximately 65 mm, which is the size of a tennis ball; however, it does not necessarily require the largest diameter site to be included in the surgical field, which is expected to make it feasible.
In our study, a corridor 2 cm in width was obtained in the oral approach, which was limited to moving an 8-mm-diameter arm with 7 degrees of freedom. It is desirable to reduce the diameter while maintaining durability. We believe that developing an arm with a narrower diameter and fewer degrees of freedom will improve operability in narrower and deeper neurosurgical fields.
Moreover, the existing forceps and scissors are not sufficiently small or delicate for neurosurgical applications, indicating a need for refinement. Should these forceps be improved, it is likely that advancements could extend from dural suturing procedures to intracranial lesion operations. Although, the level of invasiveness is similar to the current endoscopic endonasal approach and transoral approach, requiring only mucosal incisions without the need for outer incisions. The transoral approach offers good access and, if a system could be developed that secures a route or corridor to the skull base without the need for an opening device, it would likely advance further. Such a system would be beneficial whether it uses the nasal or oral route, enhancing the feasibility and scope of minimally invasive neurosurgical procedures. However, since its introduction in 1999, the da Vinci Xi has become more technically refined and safer through its application in various surgical fields. It is believed to be safer. As a crucial measure against the most significant complications, it is deemed important to have a predetermined plan for managing injuries to the internal carotid arteries in neurosurgery.
Rather than considering surgeries that can be performed using existing robots, it is essential to develop robots as tools for developing current surgeries. Although the field of neurosurgery has specific problems regarding drills, suction, and the size of tools and cameras, we want to progress towards the introduction of robots and the perfection of neurosurgical robots, keeping up with other surgical fields.
A 2-cm-wide working space was obtained in the transoral corridor when the soft palate was incised by the da Vinci Xi Surgical System. This is the first report to show the lateral reachable limitation in the coronal direction, demonstrating that the da Vinci arm can reach to the foramen ovale from the midline. In the sagittal direction, the cranial side can be manipulated to the sellar turcica, and the caudal direction can be manipulated to the second cervical vertebra. Suturing the dural defect in a deep-seated lesion using the da Vinci Xi with Dubakey forceps arms and 7-0 proline is feasible. However, developing a neurosurgery-specific robot and verifying the potential of the currently available leader-follower type robots is necessary.
NOTES
ACKNOWLEDGEMENTS
We thank Fumiaki Saito, NP and Shuhei Inada, NP for their assistance in this study.
Fig. 1.
Setup for the da Vinci Xi Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA, USA). (A) The patient was supine, positioned in the minimally extended position. (B) The da Vinci Xi Surgical System was introduced from the left side of the patient. (C) The surgeon faced the console and immersed himself in the surgical field to perform the operation. (D) A Dubakey forceps with an 8-mm diameter arm.
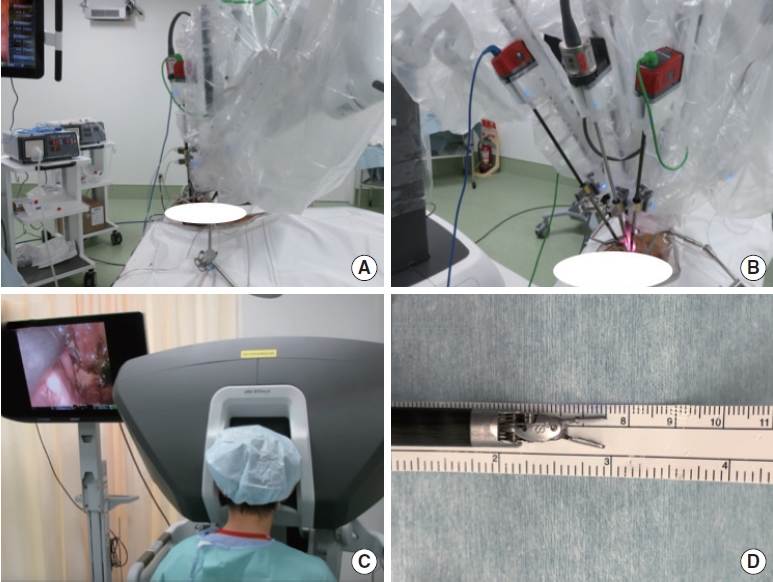
Fig. 2.
da Vinci Xi Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA, USA). approach to the skull base in the sagittal plane. (A) The first view of the transoral approach observing the soft palate and hard palate. (B) View after lateralization of the soft palate. (C) The incision of the nasopharynx for mucosal muscle flaps. (D) Exposure of clivus bone and C1 and C2 vertebrae with invert of mucosal muscle flap. (E) The endonasal endoscopic view to the nasopharynx lesion. (F) Opening the sphenoid sinus inferiorly by an angled drill. (G) Opening the sellar floor and exposure of the dura of the pituitary gland. (H) After opening the dura, the pituitary gland can be reached by the arms of the da Vinci Xi Surgical System. (I) Low-magnification view of the surgical corridor to the sellar floor using a transoral corridor.
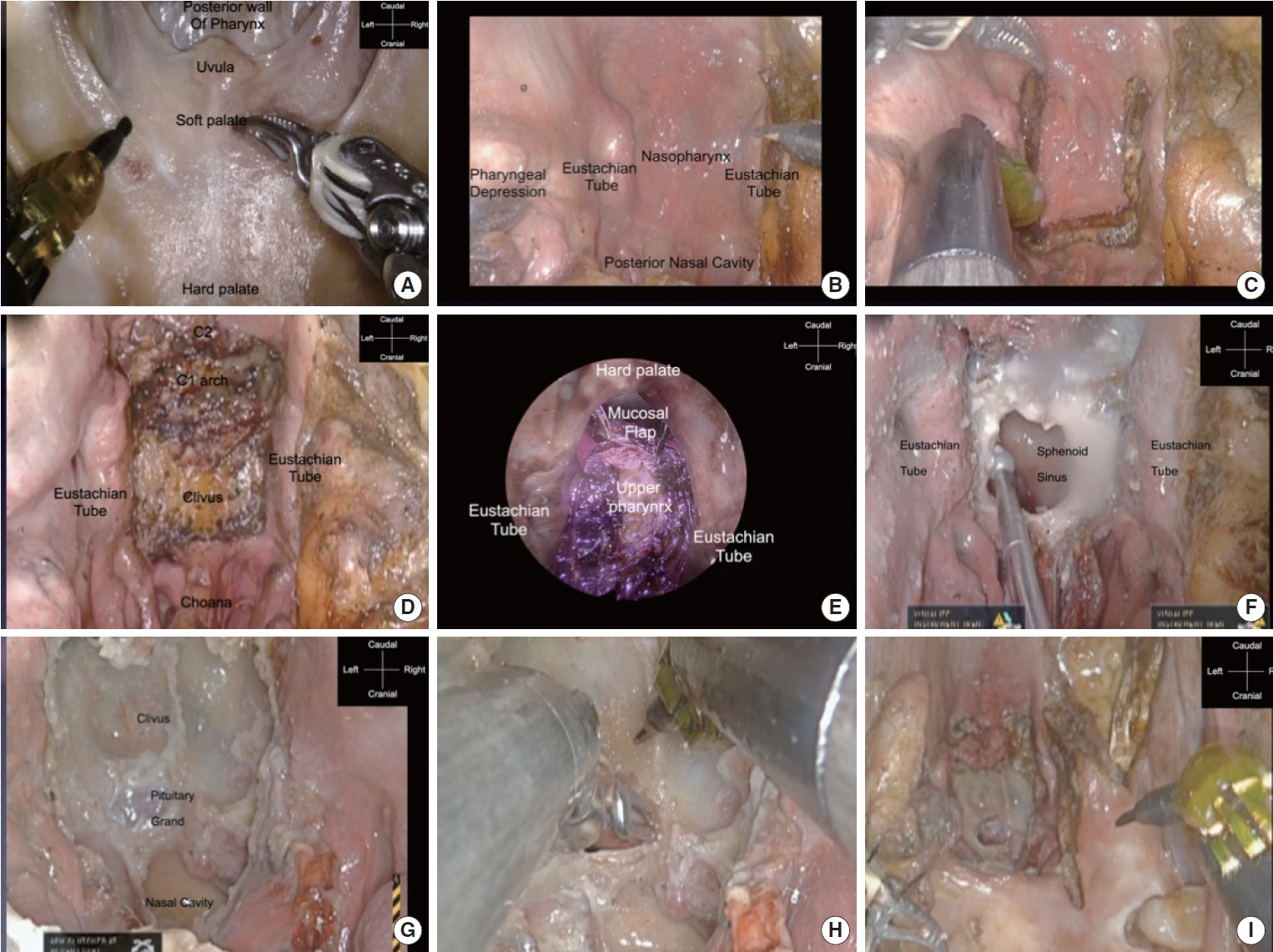
Fig. 3.
da Vinci Xi Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA, USA) approach to the skull base in the coronal plane. (A) View after lateralization of the soft palate. (B) Dissection of the tensor veli palatini and levator veli palatini muscles. (C) Identification of the ICA to avoid injury to the soft connective tissue. (D) Following the ICA to the skull base. (E) Dissection of the soft connective tissue. The ICA and foramen ovale can be identified; the white tube shows the foramen ovale. (F) The ICA going through the foramen lacerum into the intracranial space. The reachable lateral limitation is the foramen ovale. ICA, internal carotid artery.
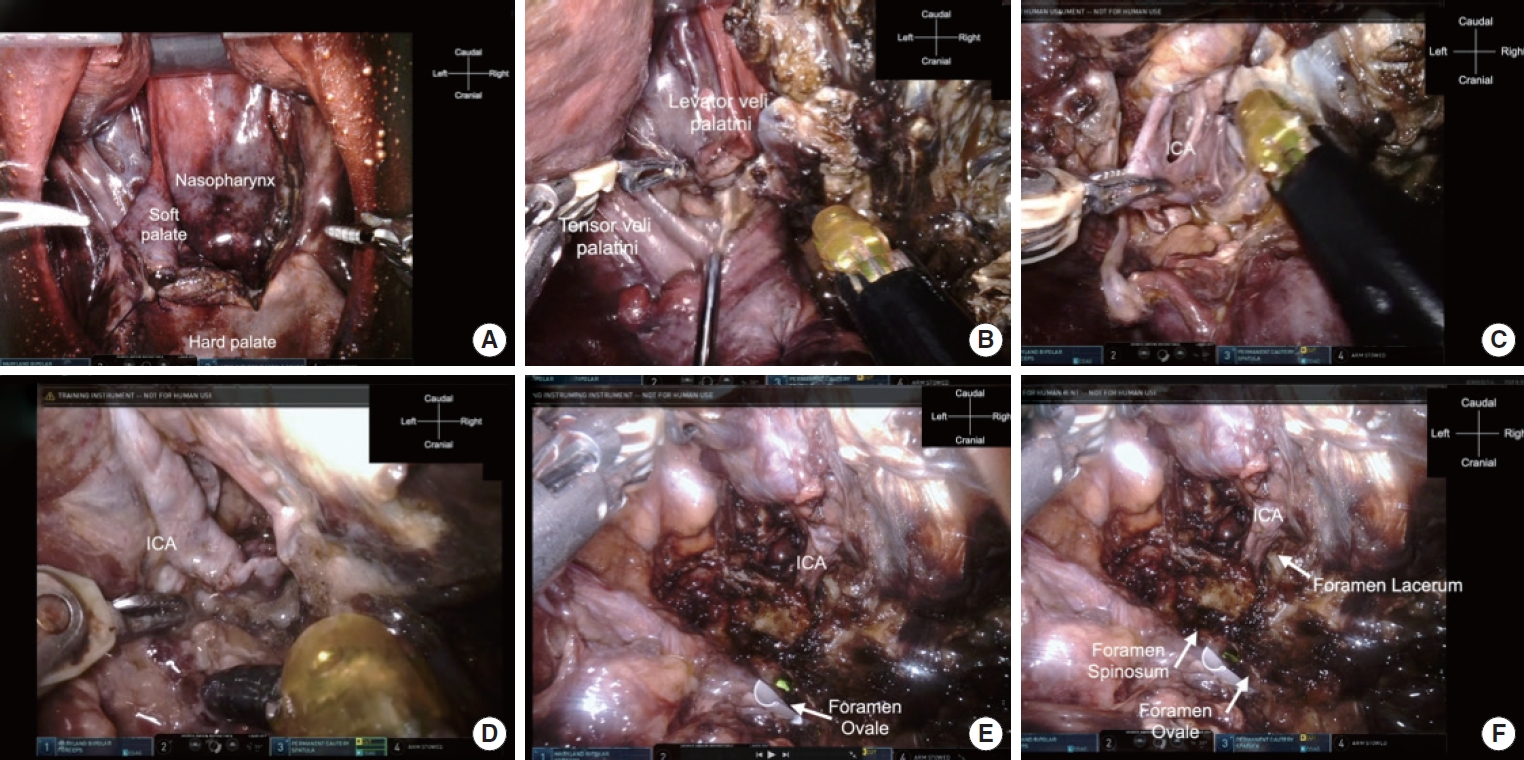
Fig. 4.
da Vinci Xi Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA, USA) approach to suturing the dura defects of the clivus. (A) Dural defect in the clivus dura. (B) Preparation of fascia (15 mm) taken from the thigh. (C) First, the top middle part of the defect is sutured, inserting the fascia into the subdural space in an inlay manner. (D) A knot is made of 7-0 proline using the Dubakey arms of the da Vinci. The system provides no haptics by hand, and surgical information is only visual. (E) The knot is tightened by robotic arms; because the working corridor is limited, the robotic arms should be used back and forth, not horizontally. (F) Suturing of the right arm, which is controlled by the surgeon’s dominant hand. (G) Suturing of the median of the bottom part with the needle held downward. (H) Suturing of the left part by an arm controlled by the surgeon’s nondominant hand without any problems. (I) Final view after the fascia was sutured to the dura mater of the clivus. The numbers (①–⑧) indicate the order in which the stitches were to be made.
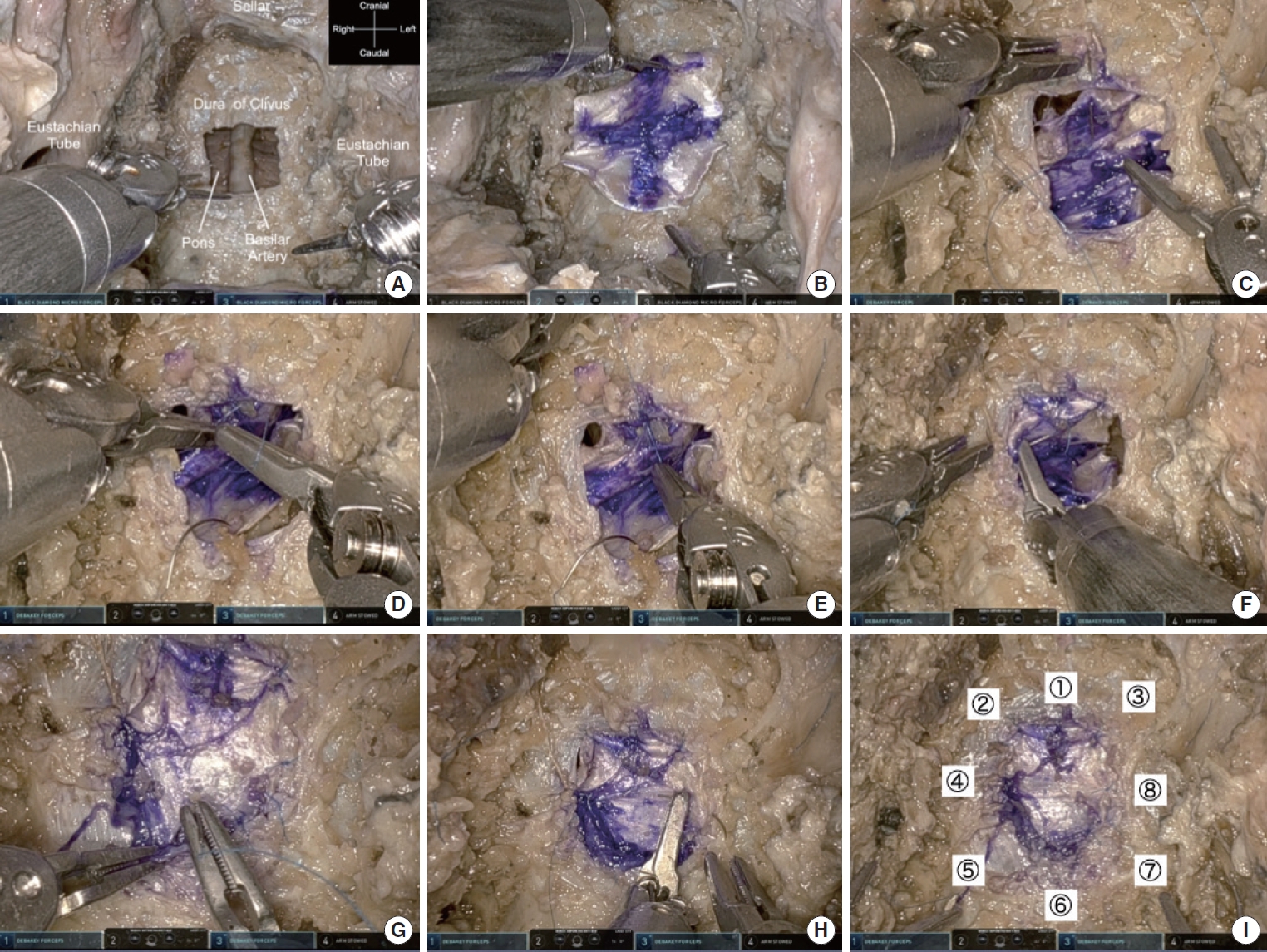
Table 1.
Time required to perform one stich, one knot, and one suture in endonasal manual suturing and robotic suturing
| Suturing | One stitch with a needle (sec) | Knot (sec) | One suture (sec) |
|---|---|---|---|
| Endonasal manual suturing | 147.6 ± 62.7 | 140.3 ± 38.3 | 459.3 ± 87.9 |
| Robotic suturing | 25.1 ± 5.6 | 31.2 ± 4.6 | 78.7 ± 15.1 |
Table 2.
Advantages and disadvantages of transnasal, transoral, and transoral robotic surgery using the da Vinci Xi Surgical System as well as the reachable range in sagittal and coronal planes
| Variable | Transnasal | Transoral | Trans oral robotic |
|---|---|---|---|
| Reachable range (sagittal) | Suprasellar-Nasoaxial line (C2) [34] | Nasoaxial line from +2 cm cranial to -3.5 cm caudal [33] | Suprasellar-Nasoaxial line (C2)-hypopharynx |
| Reachable range (coronal) | Parapharyngeal ICA [35] | 1.5 (0.9–2.5) cm from midline [33] | From midline to foramen ovale |
| Advantages | Increased illumination, narrower operative corridor, no retractor, faster recovery time | Wider operative corridor, quick access to the lesion | Fine, precise manipulation, high magnification, tremor filtration, instrument position memory, extended motion of the human hand, 3-dimensional visualization, increased instruments access |
| Disadvantages | Lesser working space, fewer instruments, nasal troubles in nasal cavity (nasal congestion, olfactory disorders, etc.) | Damage of transoral retractor (tongue compression and swelling, prolonged intubation, poor feeding), tracheal swelling, breathing difficulty), higher infection rate, splitting soft palate, swallowing difficulty | Lack of haptics, lack of drilling instruments, inconvenience of suction aspiration, low-cost performance, bulky instruments, unable to handle ICA injury quickly |
REFERENCES
1. Carrau RL, Prevedello DM, de Lara D, et al. Combined transoral robotic surgery and endoscopic endonasal approach for the resection of extensive malignancies of the skull base. Head Neck 2013;35:E351-8.


2. Dallan I, Castelnuovo P, Montevecchi F, et al. Combined transoral transnasal robotic-assisted nasopharyngectomy: a cadaveric feasibility study. Eur Arch Otorhinolaryngol 2012;269:235-9.



3. Sreenath SB, Rawal RB, Zanation AM. The combined endonasal and transoral approach for the management of skull base and nasopharyngeal pathology: a case series. Neurosurg Focus 2014;37:E2.

4. Ozer E, Waltonen J. Transoral robotic nasopharyngectomy: a novel approach for nasopharyngeal lesions. Laryngoscope 2008;118:1613-6.


5. Lee JY, O’Malley BW, Newman JG, et al. Transoral robotic surgery of craniocervical junction and atlantoaxial spine: a cadaveric study. J Neurosurg Spine 2010;12:13-8.


6. Lee JY, O’Malley BW Jr, Newman JG, et al. Transoral robotic surgery of the skull base: a cadaver and feasibility study. ORL J Otorhinolaryngol Relat Spec 2010;72:181-7.



7. Yang MS, Yoon TH, Yoon DH, et al. Robot-assisted transoral odontoidectomy: experiment in new minimally invasive technology, a cadaveric study. J Korean Neurosurg Soc 2011;49:248-51.



8. Kupferman ME, Demonte F, Levine N, et al. Feasibility of a robotic surgical approach to reconstruct the skull base. Skull Base 2011;21:79-82.



9. Hanna EY, Holsinger C, DeMonte F, et al. Robotic endoscopic surgery of the skull base: a novel surgical approach. Arch Otolaryngol Head Neck Surg 2007;133:1209-14.


10. Blanco RG, Boahene K. Robotic-assisted skull base surgery: preclinical study. J Laparoendosc Adv Surg Tech A 2013;23:776-82.


11. Hong WC, Tsai JC, Chang SD, et al. Robotic skull base surgery via supraorbital keyhole approach: a cadaveric study. Neurosurgery 2013;72 Suppl 1:33-8.

12. Bly RA, Su D, Lendvay TS, et al. Multiportal robotic access to the anterior cranial fossa: a surgical and engineering feasibility study. Otolaryngol Head Neck Surg 2013;149:940-6.




13. Dallan I, Castelnuovo P, Seccia V, et al. Combined transnasal transcervical robotic dissection of posterior skull base: feasibility in a cadaveric model. Rhinology 2012;50:165-70.


14. Quilici PJ, Wolberg H, McConnell N. Operating costs, fiscal impact, value analysis and guidance for the routine use of robotic technology in abdominal surgical procedures. Surg Endosc 2022;36:1433-43.



15. Alshowaikh K, Karpinska-Leydier K, Amirthalingam J, et al. Surgical and patient outcomes of robotic versus conventional laparoscopic hysterectomy: a systematic review. Cureus 2021;13:e16828.



16. Kazanzides P, Xia T, Baird C, et al. A cooperatively-controlled image guided robot system for skull base surgery. Stud Health Technol Inform 2008;132:198-203.

17. Bumm K, Wurm J, Rachinger J, et al. An automated robotic approach with redundant navigation for minimal invasive extended transsphenoidal skull base surgery. Minim Invasive Neurosurg 2005;48:159-64.


18. Chumnanvej S, Pillai BM, Chalongwongse S, et al. Endonasal endoscopic transsphenoidal approach robot prototype: a cadaveric trial. Asian J Surg 2021;44:345-51.


19. Gonzalez-Martinez J, Bulacio J, Thompson S, et al. Technique, results, and complications related to robot-assisted stereoelectroencephalography. Neurosurgery 2016;78:169-80.



20. Goto T, Hongo K, Ogiwara T, et al. Intelligent surgeon’s arm supporting system iArmS in microscopic neurosurgery utilizing robotic technology. World Neurosurg 2018;119:e661-5.


21. Goto T, Hongo K, Yako T, et al. The concept and feasibility of EXPERT: intelligent armrest using robotics technology. Neurosurgery 2013;72 Suppl 1:39-42.

22. Nimsky C, Rachinger J, Iro H, et al. Adaptation of a hexapod-based robotic system for extended endoscope-assisted transsphenoidal skull base surgery. Minim Invasive Neurosurg 2004;47:41-6.


23. Cabuk B, Ceylan S, Anik I, et al. A haptic guided robotic system for endoscope positioning and holding. Turk Neurosurg 2015;25:601-7.


24. Bolzoni Villaret A, Doglietto F, Carobbio A, et al. Robotic transnasal endoscopic skull base surgery: systematic review of the literature and report of a novel prototype for a hybrid system (Brescia Endoscope Assistant Robotic Holder). World Neurosurg 2017;105:875-83.


25. Friedrich DT, Sommer F, Scheithauer MO, et al. An innovate robotic endoscope guidance system for transnasal sinus and skull base surgery: proof of concept. J Neurol Surg B Skull Base 2017;78:466-72.



26. Hintschich CA, Fischer R, Seebauer C, et al. A third hand to the surgeon: the use of an endoscope holding arm in endonasal sinus surgery and well beyond. Eur Arch Otorhinolaryngol 2022;279:1891-8.




27. Pangal DJ, Cote DJ, Ruzevick J, et al. Robotic and robot-assisted skull base neurosurgery: systematic review of current applications and future directions. Neurosurg Focus 2022;52:E15.

28. Schuler PJ, Scheithauer M, Rotter N, et al. A single-port operator-controlled flexible endoscope system for endoscopic skull base surgery. HNO 2015;63:189-94.



29. Faulkner J, Naidoo R, Arora A, et al. Combined robotic transorbital and transnasal approach to the nasopharynx and anterior skull base: feasibility study. Clin Otolaryngol 2020;45:630-3.



30. Chauvet D, Hans S, Missistrano A, et al. Transoral robotic surgery for sellar tumors: first clinical study. J Neurosurg 2017;127:941-8.


31. Chauvet D, Missistrano A, Hivelin M, et al. Transoral robotic-assisted skull base surgery to approach the sella turcica: cadaveric study. Neurosurg Rev 2014;37:609-17.



32. Sano D, Shimizu A, Tateya I, et al. Current status of transoral surgery for patients with early-stage pharyngeal and laryngeal cancers in japan. Front Oncol 2021;11:804933.



33. Chan AK, Benet A, Ohya J, et al. The endoscopic transoral approach to the craniovertebral junction: an anatomical study with a clinical example. Neurosurg Focus 2016;40:E11.


- TOOLS



























