 |
 |
- Search
| Neurospine > Volume 21(1); 2024 > Article |
|
|
Abstract
Proteoglycans through their sulfated glycosaminoglycans regulate cell-matrix signaling during tissue development, regeneration, and degeneration processes. Large extracellular proteoglycans such as aggrecan, versican, and perlecan are especially important for the structural integrity of the intervertebral disc and cartilage during development. In these tissues, proteoglycans are responsible for hydration, joint flexibility, and the absorption of mechanical loads. Loss or reduction of these molecules can lead to disc degeneration and skeletal dysplasia, evident from loss of disc height or defects in skeletal development respectively. In this review, we discuss the common proteoglycans found in the disc and cartilage and elaborate on various murine models and skeletal dysplasias in humans to highlight how their absence and/or aberrant expression causes accelerated disc degeneration and developmental defects.
Low back pain (LBP) is highly prevalent as age expectancy increases, and it is the leading cause of years lived with disabilities worldwide, affecting at least 600 million people globally [1]. A widely recognized contributor to chronic LBP is the degeneration of intervertebral disc (IVD). The discs are fibrocartilaginous tissues that lie between the vertebrae of the spinal column and provide flexibility to the spine and absorbs the mechanicals loads during diurnal events [2]. During aging, the mechanical properties of the disc are often compromised due to structural changes in the tissue. Disc and cartilage function depends significantly on the integrity and composition of the extracellular matrix (ECM) which consists of intricate and finely organized networks of collagens and proteoglycans (PGs) [3]. PGs through their sulfated glycosaminoglycans (GAGs) provide an osmotic mechanism to attract water molecules into the tissue essential to accommodate compressive and tensile loads on the spine and joint tissues [4]. Aging or genetic mutations in PG genes result in reduced levels or abnormalities which impair their biological functions. This review will focus on major PGs in the IVD and cartilaginous tissue and their contribution to disc pathologies and skeletal dysplasias.
PGs generally consist of a core protein covalently attached with one or more GAG chains joined through a tetrasaccharide bridge at a serine residue. These GAGs, typically long polysaccharides with repeating disaccharide structures, are categorized into 4 groups: chondroitin sulfate/dermatan sulfate (CS/DS), heparan sulfate (HS), keratan sulfate (KS), and hyaluronic acid (HA) [5]. The biosynthesis process of GAGs, specifically HS and CS, is tightly regulated in order to maintain their constant concentration within the tissue. Alteration in HS or CS GAG levels can affect tissue development, physiological, or pathological processes [6,7]. Briefly, the process of GAG synthesis requires 5 uridine diphosphate (UDP) derived activated sugars such as UDP-glucuronic acid (GlcA), UDP-N-acetylglucosamine (GlcNAc), UDP-xylose (Xyl), UDP-galactose (Gal), and UDP-N-acetylgalactosamine (GalNAc) [5]. These sugars are transported from the cytoplasm to the Golgi apparatus where a series of glycosyltransferases assemble the tetrasaccharide bridge. GAG synthesis begins when xylosyltransferases (encoded by XT1 or 2) add Xyl to a serine residue on the core protein, followed by galactosyltransferases I (B4GALT7) and II (B3GALT6) which add 2 Gal sugars. Next, a glucuronyltransferase (B3GAT3) adds GlcA to assemble a common tetrasaccharide bridge containing GlcAβ1-3Galβ1-3Galβ1-4Xylβ1-O-Ser [5,8,9]. The subsequent addition of GalNAc by GALNACT2 or GlcNAc by EXTL3 will then initiate the commitment for either CS or HS biosynthesis [8]. The regulation of GAG biosynthesis in the context of disc biology is further discussed by Silagi et al. and others [9,10]. All GAGs, except HA, undergo a sulfation process in the Golgi where sulfotransferases catalyze sulfate donor compound 3´-phosphoadenosine-5´-phosphosulfate (PAPS) to modulate their sulfation profile [5]. Alterations in sulfation profiles can modulate the critical physiological functions of PGs in developing tissues often leading to chondrodystrophies [11]. Based on the recent classification of genetic skeletal disorders, mutations of ion transporter-related or sulfation-related genes such as SLC26A2, PAPSS2, IMPAD1, CHST3, SLC35B2, CHST14, DSE, CHST11, HS2ST1, SLC13A1 result in achondrogenesis, chondrodysplasia, Ehlers-Danlos syndrome, osteochondrodysplasia, brachydactyly, overlapping malformed digits and developmental delays, and disc degeneration [11-13]. Importantly, defects in matrix recycling by autophagy observed in lysosomal storage disorders are equally detrimental to skeletal tissues and during development [14].
It is important to mention here that PGs were previously classified either by GAG type or PG size due to their heterogeneity. To simplify PG classification, Iozzo and Schaefer proposed to categorize mammalian PGs into 4 overarching classes based on their locations: extracellular, pericellular, cell surface, and intracellular [15]. In the context of the joint, PGs are present on the cell surface and within the ECM of the growth plate, articular cartilages, and IVD. During development, these macromolecules can interact with constituent growth factors, cytokines, morphogens, and chemokines to influence cell adhesion, morphology, proliferation, migration, and differentiation [16]. Major PGs, including aggrecan, versican, perlecan, and cell surface PGs such as glypicans and syndecans, particularly syndecan-4, and a few small leucine-rich proteoglycans (SLRP), such as biglycan and decorin, fibromodulin, and lubricin are abundant in the articular cartilage and the disc to aid in tissue hydration, biomechanical function and cell signaling events (Fig. 1A and B). These will be discussed in detail below (Table 1).
ACAN encodes for aggrecan, the most abundant PG in the IVD and joint cartilage. Aggrecan bears negatively charged KS and CS GAG side chains providing cartilage with its ability to bind water for hydration and withstand large compressive loads [17]. Structurally, aggrecan consists of a core protein with 3 disulfide-linked globular regions (G1, G2, G3) with intervening extended regions between G2 and G3 [18] (Fig. 1A). Between the amino terminal G1 and G2 domains is the interglobular domain (IGD), a prominent proteolysis site that is thought to be involved in the physiological turnover of aggrecan [19]. Following the G2 domain, the core protein is decorated with approximately 30 KS chains and 100 CS chains in the CS1 and CS2 domains; these domains are responsible for the water-binding property and its function as a structural PG. The G3 domain of aggrecan resides at the carboxy-terminal and is a complex region that is required for post-transcriptional processing [19]. Aggrecan does not exist in isolation within the ECM but is instead composed of aggrecan supramolecular aggregates (Fig. 1B). These supramolecular aggregates form when multiple aggrecan molecules noncovalently attach to a link protein-bound HA filament [20]. Molecularly, the G3 domain is essential for sufficient modification of GAGs; without this, the CS-containing constructs are not secreted. In the developing skeleton, aggrecan expression is confined to chondrocytes and other cartilaginous tissues [21].
The importance of aggrecan in cartilage was first illustrated in the embryonically lethal nanomelia affecting cartilage development in chickens [22] and cartilage matrix deficiency (cmd) in mice resulting in cleft palate, dwarfism, abdominal compression, and respiratory failure after birth [23-25]. Interestingly, heterozygous cmd mice appeared normal at birth, however, age-associated skeletal defects such as dwarfism and spinal misalignment began to show at 19-month of age [26]. The underlying mechanism in lethal nanomelia avian mutation is a transversion creating a premature stop codon, truncating part of CS2 and G3 domains and significantly reducing the steady-state level of aggrecan messenger RNA (mRNA) [27]. The residual mRNA gets translated but is not secreted into the ECM [28], thus accumulating in the endoplasmic reticulum [29]. Similar to nanomelia, cmd in mice also results in reduction of aggrecan mRNA expression and secretion of aggrecan into the ECM [26]. These detrimental effects foreshadowed the outcome of ACAN mutation in humans, discussed in a later section.
Versican, another large extracellular proteoglycan encoded by VCAN, is structurally related to aggrecan, possessing a terminal domain analogous to aggrecan’s G1 and G3 regions, however it does not contain IGD, a G2 domain, and KS GAG attachment sites (Fig. 1A) [15]. To date, 5 isoforms of versican (V0–4) have been identified: the full-length versican (V0) and 3 splice variants that lack GAGα (V1), GAGβ (V2), both GAGα and GAGβ (V3), and portion of GAGβ (V4) [15]. During development, full-length versican is prominently expressed throughout the IVD [30]. As the disc matures, its expression decreases throughout but remains prominent between the lamellae of the annulus fibrosus (AF) [30,31]. Functionally, based on its structure and localization with elastin and fibulin-1, a glycoprotein that is incorporated into fibrillar ECM, versican is suggested to contribute to the organization of the disc ECM and provide structural support and resilience to mechanical forces [31]. In cartilage development, the balance of versican expression is important in mediating local transforming growth factor beta (TGF-β) in chondrocyte differentiation and digit joint formation [32-34]. Reductions in versican can compromise chondrogenesis and synovial joint development [35]. Mutations in the human versican gene result in the dominantly inherited Wagner syndrome, caused by a base substitution mutation of VCAN at exon 8 that produces less V1 and more V2 and V3 isoforms, which is associated with progressive vision loss [36].
Encoded by PRG4, proteoglycan 4 or lubricin is a mucinous glycoprotein and an atypical proteoglycan that covers the cartilage and prevents it from cartilage damage. Its structure consists of 2 adhesive nonglycosylated subdomains flanking a heavily glycosylated and mucin-like central domain region. The amino terminus possesses a somatomedin-B-like domain while the carboxyl terminus has a hemopexin domain, both of which are responsible for mediation of cell-cell and cell-matrix interactions to promote cell attachment [37,38] (Fig. 1A). The central domain mostly consists of GalNac, Gal, and NeuAc sugar groups O-linked at threonine residue, making it negatively charged and creating a strong repulsion via hydration forces [37]. Lubricin is found in the synovial joint, coated on the cartilage surface, and detected at markedly higher levels in the IVD compartments compared to other cartilage subtypes [39,40]. Lubricin deletion (Prg4-/-) in mice by excision of exon 6, removing mucin-like domain, results in age-associated accelerated synovial hyperplasia, abnormal protein deposits on the cartilage surface, and disappearance of underlying superficial zone chondrocytes ultimately contributing to joint failure [41]. Since lubricin is detected at a high level in the disc, its absence may be consequential to its integrity as discussed later.
Biglycan is a small ECM PG that is ubiquitously expressed in the articular regions, epiphyseal cartilage, vascular canals, and periosteum during development [42]. It was reported to contribute to bone growth, muscle development and regeneration, and collagen fibril assembly. Biglycan contains 2 CS or DS GAG chains at serine-glycine attachments sites in the N-terminal region (Fig. 1A). It can bind to TGF-β [43], and modulate bone morphogenetic protein (BMP)-4 to influence osteoblast differentiation and maturation in bone development [44]. Located on the X chromosome region Xq28, genetic mutation of BGN affects males more than females. Early work on BGN deficient male mice (BGN-/0) showed early onset of osteoporosis-like phenotype, skeletal abnormalities, and disc degeneration [45-47]. Closely related to biglycan is the SLRP decorin, sharing > 65% of its overall homology. It is encoded by DCN and only has one GAG chain (Fig. 1A). It is known for decorating along fibrillar collagen and regulates the association of collagen into proper fibrils protecting them from proteolysis [15] (Fig. 1B). Deletion of decorin in mice (Dcn-/-) results in fragile skin and tendons along with coarser and irregular fiber outlines without affecting bone mass [48]. In cartilage, decorin mediates matrix integrity and biomechanical functions by enhancing the linkages and assembly of aggrecan-aggrecan molecules and aggrecan-collagen II fibrils [49]. Since biglycan and decorin are highly homologous and co-expressed in tissues such as skin and bone, double deletion of biglycan and decorin (BGN-/0 Dcn-/-) mice revealed compounding effects on skin fragility, severe osteopenia, and alteration of collagen fibril structure and mechanical properties of tendons [50,51]. SLRPs fibromodulin and tenomodulin are also present in cartilage, tendon, and ligament ECM and contribute to the structural integrity of these tissues. Fibromodulin, encoded by FMOD, is a KSPG SLRP highly homologous with biglycan and decorin (Fig. 1A). It bears KS chains and binds to collagen I via residues located in leucine-rich region 11 in the convex surface of the protein core at a different region than decorin binding site [52,53]. Specifically, it interacts with triple-helical type I and II collagens [54], regulates fibrillogenesis and collagen fibril size [55], and maintains long-term tissue integrity within the knee [56] (Fig. 1B). During collagen proteolysis, fibromodulin gets cleaved by a disintegrin and metalloproteinase with thrombospondin motifs 4 and 5 (ADAMTS-4 and-5) and matrix metalloproteinase-13 (MMP-13) [57,58]. Deletion of fibromodulin in mice (Fmod-/-) results in abnormal tissue organization in the cross-sections of the tail, a larger proportion of thin collagen fibril diameter in the Achilles tendons, higher histological arthritis score, and abnormal dentin mineralization and alveolar bone formation [55,56,59-61]. Similar to BGN-/0 Dcn-/- double knockout, biglycan and fibromodulin double knockout (BGN-/0 Fmod-/-) mice have impaired collagen fibrils in the tendons. This leads to gait impairment, increased ectopic tendon ossification due to increased use of leg joints, and severe premature osteoarthritis [59]. Furthermore, these mice develop accelerated temporomandibular osteoarthritis due to accelerated chondrogenesis secondary to reduced levels of sequestered TGF-β1 in the ECM, leading to overcompensation of overactive TGF-β1 signal transduction [62]. Tenomodulin regulates tenocyte proliferation in tendons [63] and is required for proper collagen I cross-linking; deletion of tenomodulin (Tnmd-/-) in mice resulted in abnormal collagen I cross-linking and increased collagen fiber thickness and stiffness, leading to inferior endurance running performances [64,65].
Syndecans have a single-pass transmembrane protein core which includes an ectodomain bearing HS-chain or HS/CS chains, a transmembrane region, and an intracellular cytoplasmic domain. The cytoplasmic domain comprises of 2 conserved constant regions, C1 and C2, flanking a variable region, V1, that are responsible for syndecan-specific signaling [66-69] (Fig. 1A). There are 4 syndecan family members: syndecan 1-4 (encoded by SDC1-4). Sdc1 and Sdc3 are the largest family members bearing 2 CS chains and several HS chains, while Sdc2 and Sdc4 are smaller and only bear HS chains [67]. All are intrinsically disordered and dynamic, enabling them to interact with numerous ligands including fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor, BMP-2, and Indian hedgehog (IHH) [66,70]. While Sdc1-3 are found in specific tissue types, Sdc4 is ubiquitously expressed in most tissue types. Sdc4 functions as a receptor or co-receptor to ligands strengthening the duration and intensity of downstream signaling. It can also form physical connections with the ECM and activate mechanosensory signaling to influence cytoskeletal reorganization during migration and the assembly and disassembly of integrin complexes at focal adhesion sites [66,71]. During early embryo development of Xenopus laevis, gain- and loss-of-function experiments showed that the balance of Sdc4 expression was crucial in regulating the convergent-extension movement in neural tube closure and neural crest-directed migration through the noncanonical Wnt pathway by its interaction with Frizzled 7 (Fz7) and Dishevelled (Dsh) [72]. Similar to X. laevis, Sdc4 is expressed during murine development in the cranial neural folds on embryonic day 8–8.5 and is regulated by Vangl2 during neural tube closure [73]. Unlike X. laevis, Sdc4-null mice have no obvious developmental defects besides delayed wound healing, impaired angiogenesis, and defects in muscle regeneration after damage and myogenic satellite cell differentiation [74-78]. In recent years, we and others have reported that cytokines tumor necrosis factor alpha (TNF-α) and interleukin 1 beta (IL-1β) induce Sdc4 expression to mediate different musculoskeletal pathologies including rheumatoid arthritis, osteoarthritis (OA), and IVD degeneration [79-81]. Moreover, inhibition or blocking of Sdc4 under inflammatory conditions prevents exacerbation of cartilage deterioration [79].
HSPG2 encodes for the secreted perlecan, a large basement membrane HS proteoglycan presents in several ECM tissues including cartilage and the disc. The core protein of perlecan consists of 5 domains (domain I–V) with tandemly repeating modular motifs resembling “pearls-on-a-string,” 2 of which (domain I and V) possess HS GAG attachment sites [82] (Fig. 1A). Perlecan expression has been well characterized in the early stages of embryogenesis in murine models. It appears as early as E10.5 in the heart, pericardium, blood vessels, and developing vertebral cartilage [83] and is highly expressed in cartilage primordia at E15.5 [84]. Perlecan is expressed ubiquitously in muscle and bone marrow enabling homeostatic regulation of biological processes, such as the formation of cardiovascular tissue, localization of acetylcholine esterase to neuromuscular junctions [85], and formation of bone [86]. Ablation of perlecan in mice (HSPG2-/-) resulted in significant embryonic lethality due to pericardial hemorrhaging from defects in basement membranes and biomechanical deterioration of the contracting myocardium between E10–12 [87,88]. However, the few mice that survived birth died with skeletal dysplasia characterized by micromelia, narrow thorax, and craniofacial abnormalities, similar to skeletal defects of patients with lethal dyssegmental dysplasia, Silver-Handmaker type (DDSH) [88]. Furthermore, perlecan-null cartilage exhibited reduced GAGs, disorganized collagen fibrils, diminished chondrocyte proliferation, and prehypertrophic zones; all of which contributed to abnormal skeletal phenotypes similar to those of patients with thanatophoric dysplasia type I, which is caused by activating mutations in FGFR3 [88]. Since no homozygous knockout mice survived, hypomorphic HSPG2C1532Y−Neo mice were generated to study nonlethal skeletal dysplasia mimicking Schwartz-Jampel syndrome (SJS) in humans [89]. These hypomorphic HSPG2C1532Y−Neo mice have C1532Yneo mutation in domain III of perlecan, resulting in reduced perlecan expression, reduced cellular and ECM stiffness and defective pericellular matrix formation, potentially from impaired incorporation of newly synthesized ECM [89-91]. Given that perlecan was also observed in developing vertebral cartilage, another perlecan murine model lacking exon 3 (HSPG2Δ3/Δ3) was generated to study the role of HSPG2 in the disc. Perlecan exon 3 encodes perlecan domain I, and its deletion in HSPG2 exon 3 null mice resulted in a 22 kDa size reduction in the perlecan core protein [92]. As a result, HSPG2Δ3/Δ3 mice had a higher GAG content in IVDs, advanced chondrocyte hypertrophy in the cartilage, and disorganization of the growth plate [92].
The IVD is an avascular organ composed of a collagen-rich AF that encompasses the gelatinous PG-rich nucleus pulposus (NP) center; both compartments are anchored by superior and inferior endplates composed of a hyaline cartilaginous and bony endplate region. In young healthy disc tissue, the NP ECM is mainly composed of aggrecan and versican whose functions are to elevate water swelling potential and resist spinal compression. These PGs bear negatively charged CS side chains that draw in hydrated osmolytes, such as cations (Na+, Ca2+), ultimately creating a hyperosmotic environment [9]. TonEBP/NFAT5 is the only known mammalian osmo-sensitive transcription factor that plays an important role in NP cell osmoadaptation and survival within hyperosmotic environment of the disc [93-95]. AF, on the other hand, is composed of mainly collagens I and II to provide tensile strength. In degenerative disc pathologies under the aging or injury contexts, there is a marked loss of water-binding PGs and an increase of ECM remodeling that results in biochemical and biophysical changes in the disc [4]. These changes include decreased PG synthesis and increased aggrecan proteolysis by ADAMTS-4 and-5 and MMPs [96]. In addition to these proteases, cytokines including TNF-α, IL-1β, and IL-6 are important mediators of degenerative cascade under the inflammatory milieu [97].
Aggrecan is an essential component in the disc. As the disc ages, decreased aggrecan levels and functionality is observed. This is either due to a decrease in PG synthesis or an increase of PG degradation, shifting the balance from a proteoglycan-rich to collagen-rich profibrotic environment. In relatively healthy human NP tissue that correlated with gross morphologic Thompson grade of 2 (1 being the healthy and 5 being the most degenerated state), the GAG to hydroxyproline ratio was about 23:1; however, in the tissue with a Thompson grade of 4, the GAG to hydroxyproline ratio was reduced to about 5:1 [98,99]. As discussed earlier, cmd heterozygous mice cause spinal misalignment and movement problems with age due to impaired secretion or truncation of aggrecan [26]. To characterize the phenotypic and morphologic effects of aggrecan deletion on skeletal development, Lauing et al. [100] developed an ACAN mutation disease model in cmd mice (cmdbc/cmdbc) where the entire protein-coding sequence was deleted. This yielded severe skeletal defects in the limbs, ribcage, and vertebrae development with abnormal mRNA expression patterns of Col10a1, Sox9, Ihh, Ptch1, and Fgfr3 in the growth plate. However, when homozygous cmdbc was rescued with chick aggrecan transgene (cmdbc/cmdbc;Agc/+), skeletal defects were reversed by 20% in limbs and 80%, near-full reversal, in size and diameter of the ribcage and vertebrae.100 This suggests that aggrecan has a major role in regulating key growth factors during development, especially in the development of axial skeletal structures like ribcage and vertebrae. Noteworthy, these detrimental effects likely foreshadowed the outcome of ACAN mutation in humans. While the ACAN gene mutation has not been specifically reported to cause human degenerative disc disease, heterozygous ACAN mutation is associated with both idiopathic short stature and accelerated bone aging which predisposes to early onset of lumbar disc degeneration or herniation [101,102].
As mentioned, loss of aggrecan in disc pathology may be due to increased matrix degradation. Barre et al. [103] reported a significant increase in Sdc4 mRNA expression in damaged human cartilage cultured primary monolayers compared to normal cartilage tissue, suggesting Sdc4 was a likely contributor to the disorganization of cartilage and the development of OA processes. The Risbud group was the first to show enriched Sdc4 expression within the disc tissue [104] and the importance of HS synthesis in regulating matrix catabolism under an inflammatory milieu, mediating ADAMTS-5 activity to cleave aggrecan [81]. Specifically, our findings showed that cytokines TNF-α and IL-1β regulate Sdc4 expression via the nuclear factor kappa B (NF-κB)-p65/RelA dependent mechanism to activate ADAMTS-5 [81] and induce expression of MMP-3 through the mitogen-activated protein kinase–NF-κB axis [105]. Importantly, analysis of degenerated human NP tissues showed a strong correlation between Sdc4, ADAMTS-5, and cleaved aggrecan neoepitopes [81]. To investigate whether Sdc4 deletion could delay the disc degeneration, we characterized the spinal phenotype of Sdc4 null mice. Our findings show that Sdc4 deletion results in early-onset osteopenia and alters biomechanical properties in the lumbar vertebrae due to the dysregulation of osteoclastogenesis. Congruent with this finding, a previous study demonstrated that the GAG-bearing ectodomains of syndecans 1–4 suppressed osteoclast differentiation [106]. Furthermore, discs of adult Sdc4 knockout showed alterations in mature collagen crosslinking, CS content, and aggrecan turnover by aggrecanase. Interestingly, histological assessment of Sdc4 knockout mice showed subtle cellular phenotypes with NP cells containing larger vacuoles and a thicker NP cell band. Importantly, the transcriptomic analysis suggested that Sdc4 deletion downregulates genes associated with mitochondrial metabolism, autophagy, ER to Golgi protein processing, and HS biosynthesis and GAG degradation. These findings indicate that deletion of Sdc4 reduces matrix turnover which is speculated to be responsible for CS accumulation and GAG degradation in NP tissue [107]. Other investigators using Has2- and Sulf1-knockout mice have demonstrated the important of GAG function in PGs. Changes in hyaluronan synthesis and sulfation of GAG profiles can alter the structure and function of PGs. Specifically, HAS2 and SULF1 are important in HA synthesis and sulfation of GAGs, respectively, and their deletion resulted in defects of skeletal development, formation of IVDs [108], and disc degeneration [109].
Collagens are also important structural molecules that provide structural integrity to the disc. Biglycan, tenomodulin, and lubricin are all key mediators in helping collagen maintain its organization, structure, and lubrication. BGN deficient (BGN-/0) mice exhibited loss of notochordal cells at 6 months with advanced degenerative changes at 9 months. These changes were thought to have been caused by a loss of structural stability in collagens, leading to increased mechanical stress resulting in premature disc degeneration [47]. A study of Tnmd-/- mice also showed advanced degenerative changes in the NP and inner AF of the disc, increased hypertrophic-like chondrocytes and apoptosis, decreased disc height index, and smaller collagen fibrils with lower compressive stiffness. In short, decreased proliferation compounded with compromised collagen biomechanical integrity led to defects in the tissue’s ability to twist and withstand mechanical loads which subsequently increased the likelihood of degeneration and disc tears [110]. Lubricin is another important glycoprotein critical in providing joint lubrication and in aiding the endurance to mechanical strain. Lubricin knockout (Prg4-/-) mice were reported to have thinner AF, increased articular surface friction, early progressive surface damage, and increased apparent torsional modulus at L1/L2 disc [111]; all of which highlight the functional importance of lubricin as a protective barrier in the joints.
Since PGs are important in conferring mechanical, biochemical, and physical properties to tissues, mutations in genes encoding core proteins can detrimentally affect skeletal tissue development and integrity. Skeletal dysplasias noted in this review are linked to gene mutations in aggrecan (ACAN), biglycan (BGN) and perlecan (HSPG2) [12] (Table 2).
In humans, ACAN mutations result in autosomal-dominant spondyloepiphyseal dysplasia Kimberley type (SEDK) and autosomal-recessive spondylo-epi-metaphyseal dysplasia (SEMD) [112,113]. Autosomal-dominant SEDK is a skeletal dysplasia characterized by a stocky short stature and early-onset progressive joint OA. Anderson et al. first reported about SEDK identified in a multigenerational South African family of UK. white descent [114]. Subsequently, Eyre et al. [115] and Gleghorn et al. [116] performed linkage studies and identified a novel locus on chromosome 15q26.1 where a single base-pair (bp) insertion introduced a frameshift of 212 amino acids that caused a premature stop codon in ACAN, truncating aggrecan protein to lack half of the CS1 domain, the complete CS2 domain, and the G3 domain. Another autosomal-dominant mutation type led to short stature with accelerated bone maturation, early onset of OA, and craniofacial, limb, and vertebral abnormalities [117-120].
The autosomal-recessive SEMD was reported by Tompson et al. [113], describing 3 siblings with SEMD to have had extremely short stature, brachydactyly, distinct severe midface hypoplasia, short necks, barrel chests, lumbar lordosis, and macrocephaly. DNA sequence analysis of affected individuals revealed a missense mutation that predicted an amino acid substitution in the C-type lectin domain within the G3 domain [113], resulting in a reduction of aggrecan secretion [121]. A recent case reported by Fukuhara et al. [122] described an individual with SEMD caused by a heterozygous missense mutation in ACAN, however, the skeletal phenotype noted was much milder than the previous case, suggesting that mutations on different domains of ACAN can lead to different phenotypes.
Clinically, BGN mutation leads to X-linked SEMD [123] and Meester-Loeys Syndrome, a connective tissue‐arterial aneurysms disorder [124]. Both disorders are characterized by skeletal dysplasia with short stature. To date, there are only 3 cases of X-linked SEMD (SEMDX) observed in an Italian, Korean, and Indian family [125]. This SEMDX disorder was caused by a missense mutation in BGN on chromosome Xq28 and phenotypically resulted in the shortening of limbs, bowing of the legs, and lumbar lordosis.
The generation of perlecan deficient and hypomorphic mice was key to identifying HSPG2 mutations in human autosomal-recessive genetic diseases: DDSH and SJS [88,126,127]. Clinically, DDSH is a rare autosomal-recessive skeletal dysplasia with anisospondyly and micromelia caused by perlecan truncation, resulting in diminished perlecan secretion. The mutation in patients was created by 89-bp duplication of exon 34 of HSPG2 or a frameshift mutation that causes truncation in the perlecan protein core [92,126,128,129]. SJS is also a rare autosomal-recessive disorder characterized by a spectrum of abnormal neuromuscular functions and skeletal dysplasia, such as continuous contractions of muscles throughout the body including the face, abnormal spinal curvature, and shortening of the bone [92,127,130,131]. Patients with SJS survive and exhibit milder phenotypes compared to DDSH patients due to partially functional secreted perlecan [126]. These phenotypes underscore the importance of perlecan in maintaining both cartilage integrity and muscle excitability.
PGs are essential for proper skeletal development. Within the ECM, large PGs interact with growth factors and osmolytes to confer water-binding properties, tissue hydration, and bioavailability of growth factors, while smaller PGs regulate collagen fibril formation in the ECM. At the cell membrane, PGs can provide stabilization for ligand-receptor interactions, and potentiate signaling complexes that regulate growth factor sensitivity, cell migration, proliferation, and matrix adhesion. Studies have shown that gene mutations of major PGs such as aggrecan, perlecan, lubricin, biglycan, and tenomodulin result in disc degeneration and skeletal defects in murine models. These models described here could potentially be used to explore treatment modalities to restore ECM functionality during disc degeneration. In humans, proteoglycan gene mutations result in severe skeletal dysplasia, including lumbar disc herniation in some cases. Currently, there are no treatments for genetic skeletal disorders except corrective surgical procedures including osteotomy and spinal stenosis surgery.
Before we conclude, it is important to discuss a few emerging approaches to treat disc degeneration and restore the matrix function. During aging, senescent NP cells accumulate in the disc [132-134]. Senescent cells are characterized by the senescence-associated secretory phenotype factors, particularly their secretion of proinflammatory ILs and several proteases including MMPs, ADAMTSs and serine protease HTRA1. To minimize age-associated disc degeneration, restore matrix function and to promote the reparative processes, senolytic drug cocktail of Dasatinib and Quercetin (D+Q) and pentosan polysulfate, a HS biomimetic, are under investigation as attractive options [135,136]. The senolytic (D+Q) cocktail works by selectively inducing senescent cell apoptosis by targeting senescent cell antiapoptotic pathways [137,138]. It should be noted that it is technically challenging to deliver biomimetics to inner disc compartments without causing structural alterations during delivery. In contrast, systemic administration of Dasatinib and Quercetin was found to be effective in murine models and is a viable therapeutic option in preventing age-associated disc degeneration and restoring matrix quality. Similarly, long-term systemic administration of a supplement called alpha-ketoglutaric acid was reported to attenuate inflammatory- and age-associated disc degeneration by suppressing catabolic IL-6 expression and preventing JAK2/STAT3 (Janus tyrosine kinase 2/signal transducer and activator of the transcription 3) phosphorylation involved in the degenerative process [139]. In the context of injury-induced disc degeneration, Emodin, a bioactive anthraquinone compound, has been proposed as a potential therapeutic to alleviate inflammation when injected into the injury site [140,141]. This bioactive works by upregulating low-density lipoprotein receptor-related protein 1 to inhibit NF-κB mediated degradation of MMPs and ADAMTS-5, ameliorating disc degeneration and preserving aggrecan expression and functionality in vitro and in vivo [140] (See Fig. 2 for summary of these potential therapeutics). Further clinical trials and controlled studies will be required to assess the efficacy of these therapies.
NOTES
Fig. 1.
Illustration of major proteoglycans and glycoproteins in the intervertebral disc in healthy versus disease state. (A) Illustrations showing the structures of aggrecan, versican, perlecan, lubricin, syndecan-4/SDC4, decorin, biglycan, and fibromodulin. (B) In healthy state, there is an abundance of large proteoglycans that maintain tissue hydration and collagen fibril integrity. In disease state or aging, (B1) there is an elevated level of cytokines including tumor necrosis factor-α, interleukin (IL)-1β, and IL-6 which promote degenerative processes and expression of SDC4. (B2) Induction of SDC4 promotes a proteolytic event where (B3) ADAMTS-5 and matrix metalloproteinases cleave the disc extracellular matrix. ADAMTS-5, a disintegrin and metalloproteinase with thrombospondin motifs 5; HA, hyaluronic acid; HS, heparan sulfate; GAG, glycosaminoglycan; PEX, hemopexin; TMD, transmembrane domain; KS, heparan sulfate.
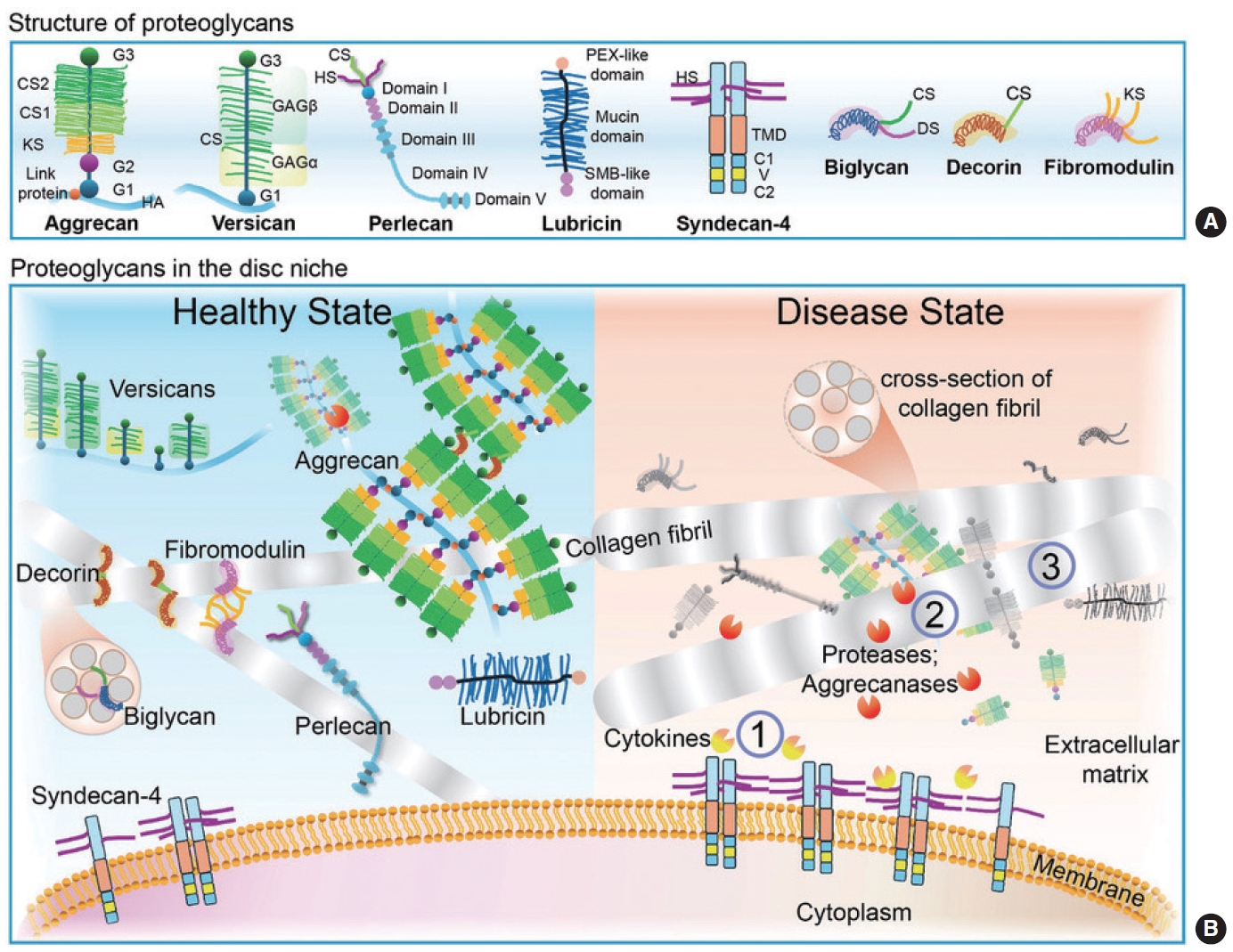
Fig. 2.
A simplified illustration showing key pathways involved in disc degeneration. Increased levels of proinflammatory cytokines tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 due to injury or aging could (1) induce degradation of lipoprotein receptor-related protein 1 (LRP1); (2) nuclear factor kappa B (NF-κB) mediated activation of p65 nuclear translocation, and subsequent promotion of SDC4-ADAMTS-5 proteolytic activity causing degrading of disc matrix; (3) activation of Janus tyrosine kinase 2/signal transducer and activator of the transcription 3 (JAK2/STAT3) signaling pathway elevating IL-6, matrix metalloproteinases (MMPs) levels, and senescent associated factors such as p21/p53; (4) increased expression of senescent cell antiapoptotic pathways (SCAPs) and senescence-associated secretory phenotype (SASP), preventing cells from secreting anabolic factors ultimately affecting tissue function. Several therapeutics such as Dasatinib and Quercetin (D+Q) senolytic drug, α-ketoglutaric acid, and Emodin in preclinical models are shown to delay or prevent degeneration by targeting SCAPs, JAK2/ STAT3 signaling axis, and LRP1 signaling pathway, respectively.
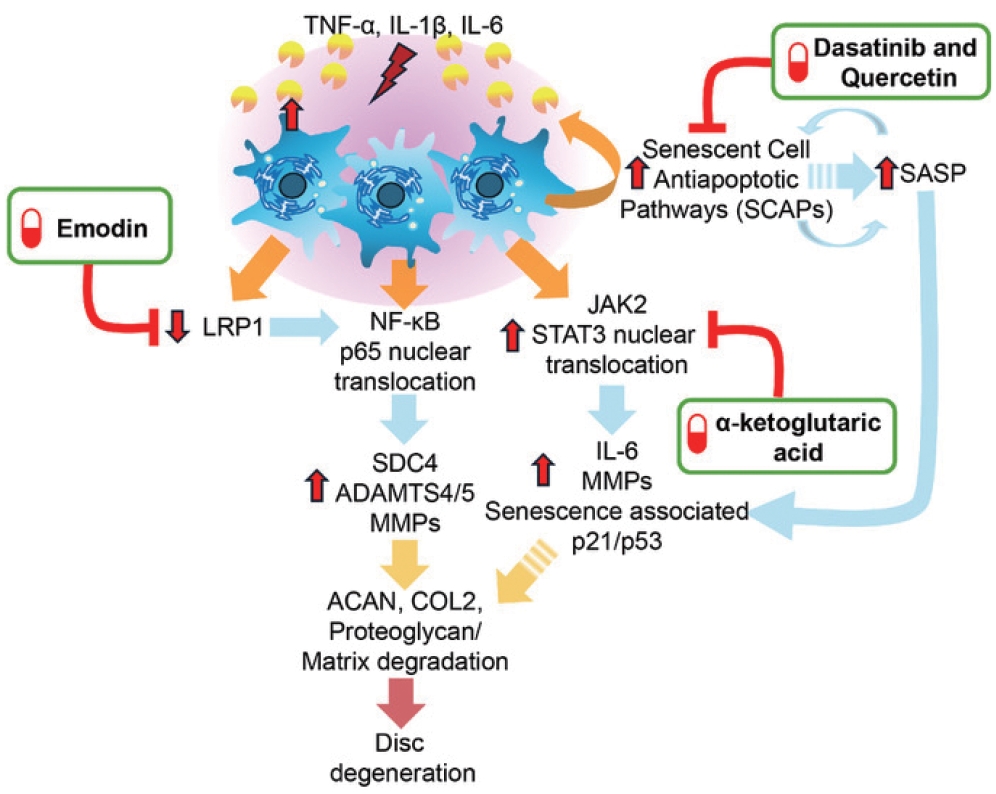
Table 1.
Animal models of proteoglycan dysfunction and mutations
| Species | Gene | Disease model/disorder | Mutation | Effect | Phenotype | References |
|---|---|---|---|---|---|---|
| Chicken | Acan | Nanomelia | Nonsense mutation | Transversion c.4537G > T: convert glutamate at 1513 to a stop codon, creating defect in CS2 and G3 domain. Truncation of aggrecan; accumulation in ER | Embryonically lethal, short-limb dwarfism, large, brachycephalic head, abnormal mandible, and maxilla | Li et al., [28] 1993; Vertel et al., [29] 1993 |
| Mouse | Acan | Cartilage matrix deficiency (cmd/cmd) | 7bp deletion in exon 5 | Deletion of B subdomain of N-terminal globular G1 domain, which binds to HA Aggrecan, results in truncation and secretion of mature aggrecan product in the matrix | Dwarfism, enlarged abdomen, short snout, cleft palate, and protruding tongue, respiratory failure related to pulmonary hypoplasia | Watanabe et al., [25] 1994 |
| Mouse | Acan | Cartilage matrix deficiency (cmdbc) | Deletion of exons 2–18 | Deletion of the globular domains G1, G2, and GAG regions KS, CS1, and C2 domains. Consequent in aggrecan truncation | Dwarfism, enlarged abdomen, short snout, cleft palate, and protruding tongue | Krugger Jr et al., [24] 1999 |
| Mouse | Bgn | Bgn-/0 | Deletion of exon 2 | Biglycan deficient | Thin dermis, low bone mass, larger irregular collagen fibril | Xu et al., [46] 1998; Chen et al., [45] 2002 |
| Mouse | Bgn; Dcn | Bgn-/0 Dcn-/- | Double deletion using Bgn-/0 and Dcn-/- | Biglycan and decorin deficient | Compounding effect with loose disorganized dermis, larger highly irregular collagen fibrils in tendons | Robinson et al., [51] 2017 |
| Mouse | Dcn | Dcn-/- | Deletion of exon 2 | Decorin deficient | Thin dermis, low bone mass, larger irregular collagen fibril | Danielson et al., [48] 1997 |
| Mouse | Fmod | Fmod-/- | Deletion of exon 2 | Fibromodulin deficient | Abnormal tissue organization, thin collagen fibril diameter; abnormal dentin mineralization and alveolar bone formation | Gill et al., [56] 2002; Ameye et al., [59] 2002; Goldberg et al., [61] 2009 |
| Mouse | Hspg2 | Hspg2-/- | Deletion of exon 6 | Mutant perlecan allele is transcribed, truncated protein could not be detected with a domain I–specific polyclonal antibody. Truncated form of domain I is not properly folded and is consequently degraded intracellularly as soon as it is translated | Homozygous mice died between E10–12 and perinatally. Embryos develop severe defect in cartilage, a tissue that lacks basement membranes. Reduced fibrillar collagen network shortened collagen fibers, and increased expression of cartilage ECM genes. Expansion of neuroepithelium, neuronal ectopias, and exencephaly | Costell et al., [87] 1999 |
| Mouse | Hspg2 | Hspg2C1532Y−Neo | Substitution mutation | G-to-A substitution at Hspg2 nucleotide 4595, change amino acid from C to Y at residue 1532. Disrupt disulfide bond formation within perlecan domain III, thus affect perlecan conformation | Reduction of perlecan. Altered matrix organization and stiffness as function of age and genotype. Short stature, impaired mineralization, misshapen bones, osteoarthritis-like joint dysplasias and myotonia. Associated with SJS disease in humans | Rodgers et al., [89] 2007; Xu et al., [90] 2016; Ocken et al., [91] 2020 |
| Mouse | Hspg2 | Hspg2Δ3/Δ3 | Deletion of exon 3 | Perlecan is devoid of ~20 kDa of domain I of its core protein and the GAG chains normally attached to this region; there was no detectable GAG substitution in domain V | GAGs accumulation in the nucleus pulposus, annulus fibrosus, and vertebral growth plates. Advanced chondrocyte hypertrophy and exostosis-like, ectopic bone formation at the cartilaginous endplate region | Shu et al., [92] 2019 |
| Mouse | Prg4 | Prg4-/- | Deletion of exon 6 | Removal of mucin-like domain; lubricin deficient | Accelerated synovial hyperplasia, disappearance of superficial zone chondrocytes. Increase articular surface friction, increase apparent torsional modulus in L1/2 disc, thinner AF | Rhee et al., [41] 2005; Teeple et al., [111] 2015 |
| Mouse | Sdc4 | Sdc4-/- | Deletion of exon 2-part of 4 | Deletion of ectodomain with three putative glycosaminoglycan attachment sites | Impaired wound healing and angiogenesis. Syndecan 4 regulates ADAMTS-5 and MMP3 under inflammatory milieu to degrade aggrecan. Essential for endochondral ossification to repair fracture healing. Exhibits smaller muscle fiber, defect in muscle regeneration after damage and myogenic satellite cell differentiation | Ishiguro et al., [74] 2000; Echtermeyer et al., [75] 2001; Cornelison et al., [77] 2004; Bertrand et al., [76] 2013; Rønning et al., [78] 2020; De Rossi et al., [76] 2021 |
| Mouse | Tnmd | Tnmd-/- | Deletion of exon 1 | Tenomodulin deficient | Advanced degenerative of the disc, increase hypertrophic-like chondrocytes, apoptosis, small collagen fibrils with low compressive stiffness. Abnormal collagen I cross-linking, inferior endurance running performance | Lin et al., [110] 2020; Docheva et al., [63] 2005; Dex et al., [64] 2017 |
| Mouse | Vcan | Prx1-Vcan | Deletion of exon 2 | Deletion of G1 domain; affect biosynthesis of versican | Distorted digits, tilted Joint surface and delayed cartilage development | Choocheep et al., [33] 2010; Higuchi et al., [34] 2021 |
Table 2.
Skeletal dysplasias caused by proteoglycan dysfunction and mutation
| Species | Gene | Disease model/disorder | Mutation | Effect | Phenotype | References |
|---|---|---|---|---|---|---|
| Human | ACAN | Spondyloepiphyseal dysplasia, Kimberley type (SEDK) | Frameshift mutation; autosomal dominant | A single-base-pair insertion, within the variable repeat region of exon 12, introduce a 212 amino acid frameshift followed by premature stop codon. Lack half of the CS1 domain, the complete CS2 domain, and the G3 domain, resulting in abnormally short aggrecan protein | Short stature and early development of osteoarthritis, especially in the knees, ankles, and hips. Variation of mutation also results in short stature with accelerated bone maturation, early onset of osteoarthritis, and craniofacial, limb, and vertebral abnormalities | Anderson et al., [114] 1990; Eyre et al., [115] 2005; Gleghorn et al., [116] 2005; Nilsson et al., [117] 2014; Kim et al., [118] 2022; Karatas et al., [119] 2023; Huang et al., [120] 2023 |
| Human | ACAN | Spondyloepimetaphy-seal dysplasia (SEMD) | Missense mutation; autosomal dominant | Point mutation c.6799G > A p.Asp2267Asn amino acid substitution affected a highly conserved residue that contributes to the structure of the C-type lectin domain within the G3 domain of the protein, in part by coordinating binding of one of three calcium ions important for its structure | Heterogeneous group of disorders defined by the combination of vertebral, epiphyseal, and metaphyseal anomalies. Short stature, brachydactyly, distinct severe midface hypoplasia, short necks, barrel chests, lumbar lordosis, and macrocephaly | Tompson et al., [113] 2009; Fukuhara et al., [122] 2019; Stattin et al., [121] 2022 |
| Human | BGN | X-linked SEMD | Spectrum of mutation: insertion/ deletion, missense, and nonsense | Mutation in BGN on chromosome Xq28. Point mutation c.439A > G (p.Lys147Glu) in Korean family; c.776G > T (p.Gly259Val) in the Italian family; and c.439A > G (p.Lys147Glu) mutation in the Indian patient | SEMDX were reported in three different ethnic background. Characterized by short stature with shortening of limbs, bowing of the legs, and lumbar lordosis | Camera et al., [123] 1994; Cho et al., [125] 2016; Meester et al., [124] 2017 |
| Human | HSPG2 | Dyssegmental dysplasia, Silver-Handmaker type (DDSH) | Frameshift mutation; lethal autosomal recessive | 89-bp duplication of exon 34 of HSPG2; one unrelated case with 89-bp duplication compound with point mutation that results in skipping of entire exons 52 and 73. Homozygous 4-bp deletion in exon 31 causes frameshift mutation that results in truncation in the perlecan protein core | Diminished perlecan secretion. Dwarfism, anisospondyly and micromelia, small mouth, small chest, die shortly from respiratory insufficiency | Arikawa-Hirasawa et al., [126] 2001; Rieubland et al., [128] 2010; Ladhani et al., [129] 2013 |
| Human | HSPG2 | Schwartz-Jampel syndrome (SJS) | Spectrum of mutations: insertion/ deletion, missense, and nonsense. Autosomal recessive | Spectrum of mutations affecting perlecan domain II, III, IV, V. For example: Point mutation c.2746C>T on exon 22 from one parent results in premature stop codon (p.R916X), reducing perlecan mRNA level by 36.3%. Another case found c.1125C > G; p.Cys375Trp of HSPG2 affect perlecan domain II | Continuous contractions of muscles throughout the body including the face, abnormal spinal curvature and shortening of the bone. Narrow eye openings (blepharophimosis) and pursed lips | Bauché et al., [130] 2013; Lin et al., [131] 2021 |
REFERENCES
1. Collaborators GBDLBP. Global, regional, and national burden of low back pain, 1990-2020, its attributable risk factors, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol 2023;5:e316-29.


2. Shapiro IM, Vresilovic EJ, Risbud MV. Is the spinal motion segment a diarthrodial polyaxial joint: what a nice nucleus like you doing in a joint like this? Bone 2012;50:771-6.



3. Karamanos NK, Theocharis AD, Piperigkou Z, et al. A guide to the composition and functions of the extracellular matrix. FEBS J 2021;288:6850-912.



4. Roberts S, Evans H, Trivedi J, et al. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am 2006;88 Suppl 2:10-4.


5. Casale J, Crane JS. Biochemistry, glycosaminoglycans. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
6. Basu A, Patel NG, Nicholson ED, et al. Spatiotemporal diversity and regulation of glycosaminoglycans in cell homeostasis and human disease. Am J Physiol Cell Physiol 2022;322:C849-64.



7. Wang Q, Chi L. The Alterations and Roles of Glycosaminoglycans in Human Diseases. Polymers (Basel) 2022;14:5014.



8. Sammon D, Krueger A, Busse-Wicher M, et al. Molecular mechanism of decision-making in glycosaminoglycan biosynthesis. Nat Commun 2023;14:6425.




9. Silagi ES, Shapiro IM, Risbud MV. Glycosaminoglycan synthesis in the nucleus pulposus: dysregulation and the pathogenesis of disc degeneration. Matrix Biol 2018;71-72:368-79.



10. Merry CLR, Lindahl U, Couchman J, et al. Proteoglycans and sulfated glycosaminoglycans. In: Varki A, Cummings RD, Esko JD, et al., eds. Essentials of glycobiology. 4th ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2022. p. 217-32.
11. Paganini C, Gramegna Tota C, Superti-Furga A, et al. Skeletal dysplasias caused by sulfation defects. Int J Mol Sci 2020;21:2710.



12. Unger S, Ferreira CR, Mortier GR, et al. Nosology of genetic skeletal disorders: 2023 revision. Am J Med Genet A 2023;191:1164-209.



13. Bjornsdottir G, Stefansdottir L, Thorleifsson G, et al. Rare SLC13A1 variants associate with intervertebral disc disorder highlighting role of sulfate in disc pathology. Nat Commun 2022;13:634.


14. Madhu V, Guntur AR, Risbud MV. Role of autophagy in intervertebral disc and cartilage function: implications in health and disease. Matrix Biol 2021;100-101:207-20.



15. Iozzo RV, Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol 2015;42:11-55.



16. Elfenbein A, Simons M. Auxiliary and autonomous proteoglycan signaling networks. Methods Enzymol 2010;480:3-31.



17. Roughley PJ, Mort JS. The role of aggrecan in normal and osteoarthritic cartilage. J Exp Orthop 2014;1:8.




18. Sandy JD, Flannery CR, Boynton RE, et al. Isolation and characterization of disulfide-bonded peptides from the three globular domains of aggregating cartilage proteoglycan. J Biol Chem 1990;265:21108-13.


21. Mundlos S, Meyer R, Yamada Y, et al. Distribution of cartilage proteoglycan (aggrecan) core protein and link protein gene expression during human skeletal development. Matrix 1991;11:339-46.


23. Rittenhouse E, Dunn LC, Cookingham J, et al. Cartilage matrix deficiency (cmd): a new autosomal recessive lethal mutation in the mouse. J Embryol Exp Morphol 1978;43:71-84.



24. Krueger RC Jr, Kurima K, Schwartz NB. Completion of the mouse aggrecan gene structure and identification of the defect in the cmd-Bc mouse as a near complete deletion of the murine aggrecan gene. Mamm Genome 1999;10:1119-25.



25. Watanabe H, Kimata K, Line S, et al. Mouse cartilage matrix deficiency (cmd) caused by a 7 bp deletion in the aggrecan gene. Nat Genet 1994;7:154-7.



26. Watanabe H, Nakata K, Kimata K, et al. Dwarfism and ageassociated spinal degeneration of heterozygote cmd mice defective in aggrecan. Proc Natl Acad Sci U S A 1997;94:6943-7.



27. Stirpe NS, Argraves WS, Goetinck PF. Chondrocytes from the cartilage proteoglycan-deficient mutant, nanomelia, synthesize greatly reduced levels of the proteoglycan core protein transcript. Dev Biol 1987;124:77-81.


28. Li H, Schwartz NB, Vertel BM. cDNA cloning of chick cartilage chondroitin sulfate (aggrecan) core protein and identification of a stop codon in the aggrecan gene associated with the chondrodystrophy, nanomelia. J Biol Chem 1993;268:23504-11.


29. Vertel BM, Walters LM, Grier B, et al. Nanomelic chondrocytes synthesize, but fail to translocate, a truncated aggrecan precursor. J Cell Sci 1993;104(Pt 3):939-48.



30. Smith SM, Whitelock JM, Iozzo RV, et al. Topographical variation in the distributions of versican, aggrecan and perlecan in the foetal human spine reflects their diverse functional roles in spinal development. Histochem Cell Biol 2009;132:491-503.



31. Melrose J, Ghosh P, Taylor TK. A comparative analysis of the differential spatial and temporal distributions of the large (aggrecan, versican) and small (decorin, biglycan, fibromodulin) proteoglycans of the intervertebral disc. J Anat 2001;198:3-15.




32. Lorda-Diez CI, Montero JA, Martinez-Cue C, et al. Transforming growth factors beta coordinate cartilage and tendon differentiation in the developing limb mesenchyme. J Biol Chem 2009;284:29988-96.


33. Choocheep K, Hatano S, Takagi H, et al. Versican facilitates chondrocyte differentiation and regulates joint morphogenesis. J Biol Chem 2010;285:21114-25.



34. Higuchi T, Suzuki D, Watanabe T, et al. Versican contributes to ligament formation of knee joints. PLoS One 2021;16:e0250366.



35. Shepard JB, Gliga DA, Morrow AP, et al. Versican knockdown compromises chondrogenesis in the embryonic chick limb. Anat Rec (Hoboken) 2008;291:19-27.


36. Kloeckener-Gruissem B, Bartholdi D, Abdou MT, et al. Identification of the genetic defect in the original Wagner syndrome family. Mol Vis 2006;12:350-5.

37. Lee Y, Choi J, Hwang NS. Regulation of lubricin for functional cartilage tissue regeneration: a review. Biomater Res 2018;22:9.




38. Deng G, Curriden SA, Hu G, et al. Plasminogen activator inhibitor-1 regulates cell adhesion by binding to the somatomedin B domain of vitronectin. J Cell Physiol 2001;189:23-33.


39. Swann DA, Silver FH, Slayter HS, et al. The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids. Biochem J 1985;225:195-201.




40. Onnerfjord P, Khabut A, Reinholt FP, et al. Quantitative proteomic analysis of eight cartilaginous tissues reveals characteristic differences as well as similarities between subgroups. J Biol Chem 2012;287:18913-24.



41. Rhee DK, Marcelino J, Baker M, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest 2005;115:622-31.



42. Chen XD, Allen MR, Bloomfield S, et al. Biglycan-deficient mice have delayed osteogenesis after marrow ablation. Calcif Tissue Int 2003;72:577-82.



43. Hildebrand A, Romaris M, Rasmussen LM, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J 1994;302(Pt 2):527-34.


44. Chen XD, Fisher LW, Robey PG, et al. The small leucinerich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. FASEB J 2004;18:948-58.



45. Chen XD, Shi S, Xu T, et al. Age-related osteoporosis in biglycan-deficient mice is related to defects in bone marrow stromal cells. J Bone Miner Res 2002;17:331-40.



46. Xu T, Bianco P, Fisher LW, et al. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet 1998;20:78-82.



47. Furukawa T, Ito K, Nuka S, et al. Absence of biglycan accelerates the degenerative process in mouse intervertebral disc. Spine (Phila Pa 1976) 2009;34:E911-7.



48. Danielson KG, Baribault H, Holmes DF, et al. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol 1997;136:729-43.




49. Han B, Li Q, Wang C, et al. Decorin Regulates the aggrecan network integrity and biomechanical functions of cartilage extracellular matrix. ACS Nano 2019;13:11320-33.



50. Young MF, Bi Y, Ameye L, et al. Biglycan knockout mice: new models for musculoskeletal diseases. Glycoconj J 2002;19:257-62.


51. Robinson KA, Sun M, Barnum CE, et al. Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons. Matrix Biol 2017;64:81-93.



52. Hedbom E, Heinegard D. Binding of fibromodulin and decorin to separate sites on fibrillar collagens. J Biol Chem 1993;268:27307-12.


53. Kalamajski S, Oldberg A. Fibromodulin binds collagen type I via Glu-353 and Lys-355 in leucine-rich repeat 11. J Biol Chem 2007;282:26740-5.


54. Hedbom E, Heinegard D. Interaction of a 59-kDa connective tissue matrix protein with collagen I and collagen II. J Biol Chem 1989;264:6898-905.


55. Svensson L, Aszodi A, Reinholt FP, et al. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem 1999;274:9636-47.


56. Gill MR, Oldberg A, Reinholt FP. Fibromodulin-null murine knee joints display increased incidences of osteoarthritis and alterations in tissue biochemistry. Osteoarthritis Cartilage 2002;10:751-7.


57. Heathfield TF, Onnerfjord P, Dahlberg L, et al. Cleavage of fibromodulin in cartilage explants involves removal of the N-terminal tyrosine sulfate-rich region by proteolysis at a site that is sensitive to matrix metalloproteinase-13. J Biol Chem 2004;279:6286-95.


58. Shu CC, Flannery CR, Little CB, et al. Catabolism of fibromodulin in developmental rudiment and pathologic articular cartilage demonstrates novel roles for MMP-13 and ADAMTS-4 in C-terminal Processing of SLRPs. Int J Mol Sci 2019;20:579.



59. Ameye L, Aria D, Jepsen K, et al. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J 2002;16:673-80.



60. Goldberg M, Marchadier A, Vidal C, et al. Differential effects of fibromodulin deficiency on mouse mandibular bones and teeth: a micro-CT time course study. Cells Tissues Organs 2011;194:205-10.




61. Goldberg M, Ono M, Septier D, et al. Fibromodulin-deficient mice reveal dual functions for fibromodulin in regulating dental tissue and alveolar bone formation. Cells Tissues Organs 2009;189:198-202.




62. Embree MC, Kilts TM, Ono M, et al. Biglycan and fibromodulin have essential roles in regulating chondrogenesis and extracellular matrix turnover in temporomandibular joint osteoarthritis. Am J Pathol 2010;176:812-26.



63. Docheva D, Hunziker EB, Fassler R, et al. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol 2005;25:699-705.




64. Dex S, Alberton P, Willkomm L, et al. Tenomodulin is required for tendon endurance running and collagen I fibril adaptation to mechanical load. EBioMedicine 2017;20:240-54.



65. Delgado Caceres M, Angerpointner K, Galler M, et al. Tenomodulin knockout mice exhibit worse late healing outcomes with augmented trauma-induced heterotopic ossification of Achilles tendon. Cell Death Dis 2021;12:1049.


67. Gondelaud F, Ricard-Blum S. Structures and interactions of syndecans. FEBS J 2019;286:2994-3007.



68. Gopal S, Arokiasamy S, Pataki C, et al. Syndecan receptors: pericellular regulators in development and inflammatory disease. Open Biol 2021;11:200377.




69. Binch ALA, Shapiro IM, Risbud MV. Syndecan-4 in intervertebral disc and cartilage: saint or synner? Matrix Biol 2016;52-54:355-62.



70. Ricard-Blum S, Couchman JR. Conformations, interactions and functions of intrinsically disordered syndecans. Biochem Soc Trans 2023;51:1083-96.



71. Chronopoulos A, Thorpe SD, Cortes E, et al. Syndecan-4 tunes cell mechanics by activating the kindlin-integrinRhoA pathway. Nat Mater 2020;19:669-78.




72. Munoz R, Moreno M, Oliva C, et al. Syndecan-4 regulates non-canonical Wnt signalling and is essential for convergent and extension movements in Xenopus embryos. Nat Cell Biol 2006;8:492-500.



73. Escobedo N, Contreras O, Munoz R, et al. Syndecan 4 interacts genetically with Vangl2 to regulate neural tube closure and planar cell polarity. Development 2013;140:3008-17.




74. Ishiguro K, Kadomatsu K, Kojima T, et al. Syndecan-4 deficiency impairs focal adhesion formation only under restricted conditions. J Biol Chem 2000;275:5249-52.


75. Echtermeyer F, Streit M, Wilcox-Adelman S, et al. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest 2001;107:R9-14.



76. Bertrand J, Stange R, Hidding H, et al. Syndecan 4 supports bone fracture repair, but not fetal skeletal development, in mice. Arthritis Rheum 2013;65:743-52.


77. Cornelison DD, Wilcox-Adelman SA, Goetinck PF, et al. Essential and separable roles for Syndecan-3 and Syndecan-4 in skeletal muscle development and regeneration. Genes Dev 2004;18:2231-6.



78. Ronning SB, Carlson CR, Aronsen JM, et al. Syndecan4(-/-) mice have smaller muscle fibers, increased Akt/ mTOR/S6K1 and Notch/HES-1 pathways, and alterations in extracellular matrix components. Front Cell Dev Biol 2020;8:730.


79. Godmann L, Bollmann M, Korb-Pap A, et al. Antibodymediated inhibition of syndecan-4 dimerisation reduces interleukin (IL)-1 receptor trafficking and signalling. Ann Rheum Dis 2020;79:481-9.


80. Echtermeyer F, Bertrand J, Dreier R, et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med 2009;15:1072-6.



81. Wang J, Markova D, Anderson DG, et al. TNF-alpha and IL-1beta promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem 2011;286:39738-49.


82. Paulsson M, Yurchenco PD, Ruben GC, et al. Structure of low density heparan sulfate proteoglycan isolated from a mouse tumor basement membrane. J Mol Biol 1987;197:297-313.


83. Handler M, Yurchenco PD, Iozzo RV. Developmental expression of perlecan during murine embryogenesis. Dev Dyn 1997;210:130-45.


84. French MM, Smith SE, Akanbi K, et al. Expression of the heparan sulfate proteoglycan, perlecan, during mouse embryogenesis and perlecan chondrogenic activity in vitro. J Cell Biol 1999;145:1103-15.




85. Zoeller JJ, McQuillan A, Whitelock J, et al. A central function for perlecan in skeletal muscle and cardiovascular development. J Cell Biol 2008;181:381-94.




86. Lowe DA, Lepori-Bui N, Fomin PV, et al. Deficiency in perlecan/HSPG2 during bone development enhances osteogenesis and decreases quality of adult bone in mice. Calcif Tissue Int 2014;95:29-38.




87. Costell M, Gustafsson E, Aszodi A, et al. Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol 1999;147:1109-22.




88. Arikawa-Hirasawa E, Watanabe H, Takami H, et al. Perlecan is essential for cartilage and cephalic development. Nat Genet 1999;23:354-8.



89. Rodgers KD, Sasaki T, Aszodi A, et al. Reduced perlecan in mice results in chondrodysplasia resembling SchwartzJampel syndrome. Hum Mol Genet 2007;16:515-28.


90. Xu X, Li Z, Leng Y, et al. Knockdown of the pericellular matrix molecule perlecan lowers in situ cell and matrix stiffness in developing cartilage. Dev Biol 2016;418:242-7.



91. Ocken AR, Ku MM, Kinzer-Ursem TL, et al. Perlecan knockdown significantly alters extracellular matrix composition and organization during cartilage development. Mol Cell Proteomics 2020;19:1220-35.



92. Shu CC, Smith SM, Little CB, et al. Elevated hypertrophy, growth plate maturation, glycosaminoglycan deposition, and exostosis formation in the Hspg2 exon 3 null mouse intervertebral disc. Biochem J 2019;476:225-43.



93. Tsai TT, Danielson KG, Guttapalli A, et al. TonEBP/OREBP is a regulator of nucleus pulposus cell function and survival in the intervertebral disc. J Biol Chem 2006;281:25416-24.


94. Gajghate S, Hiyama A, Shah M, et al. Osmolarity and intracellular calcium regulate aquaporin2 expression through TonEBP in nucleus pulposus cells of the intervertebral disc. J Bone Miner Res 2009;24:992-1001.




95. Tessier S, Madhu V, Johnson ZI, et al. NFAT5/TonEBP controls early acquisition of notochord phenotypic markers, collagen composition, and sonic hedgehog signaling during mouse intervertebral disc embryogenesis. Dev Biol 2019;455:369-81.



96. Wang WJ, Yu XH, Wang C, et al. MMPs and ADAMTSs in intervertebral disc degeneration. Clin Chim Acta 2015;448:238-46.


97. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol 2014;10:44-56.




98. Mwale F, Roughley P, Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater 2004;8:58-63. discussion 63-4.


99. Benneker LM, Heini PF, Anderson SE, et al. Correlation of radiographic and MRI parameters to morphological and biochemical assessment of intervertebral disc degeneration. Eur Spine J 2005;14:27-35.




100. Lauing KL, Cortes M, Domowicz MS, et al. Aggrecan is required for growth plate cytoarchitecture and differentiation. Dev Biol 2014;396:224-36.



101. Kim TY, Jang KM, Keum CW, et al. Identification of a heterozygous ACAN mutation in a 15-year-old boy with short stature who presented with advanced bone age: a case report and review of the literature. Ann Pediatr Endocrinol Metab 2020;25:272-6.




102. Dateki S, Nakatomi A, Watanabe S, et al. Identification of a novel heterozygous mutation of the Aggrecan gene in a family with idiopathic short stature and multiple intervertebral disc herniation. J Hum Genet 2017;62:717-21.



103. Barre PE, Redini F, Boumediene K, et al. Semiquantitative reverse transcription-polymerase chain reaction analysis of syndecan-1 and -4 messages in cartilage and cultured chondrocytes from osteoarthritic joints. Osteoarthritis Cartilage 2000;8:34-43.


104. Fujita N, Hirose Y, Tran CM, et al. HIF-1-PHD2 axis controls expression of syndecan 4 in nucleus pulposus cells. FASEB J 2014;28:2455-65.




105. Wang X, Wang H, Yang H, et al. Tumor necrosis factor-alpha- and interleukin-1beta-dependent matrix metalloproteinase-3 expression in nucleus pulposus cells requires cooperative signaling via syndecan 4 and mitogen-activated protein kinase-NF-kappaB axis: implications in inflammatory disc disease. Am J Pathol 2014;184:2560-72.


106. Kim JM, Lee K, Kim MY, et al. Suppressive effect of syndecan ectodomains and N-desulfated heparins on osteoclastogenesis via direct binding to macrophage-colony stimulating factor. Cell Death Dis 2018;9:1119.




107. Sao K, Risbud MV. SDC4 deletion perturbs intervertebral disc matrix homeostasis and promotes early osteopenia in the aging spine. bioRxiv 2023:2023.09.24.559195https://doi.org/10.1101/2023.09.24.559195.

108. Roughley PJ, Lamplugh L, Lee ER, et al. The role of hyaluronan produced by Has2 gene expression in development of the spine. Spine (Phila Pa 1976) 2011;36:E914-20.


109. Otsuki S, Alvarez-Garcia O, Lotz MK, et al. Role of heparan sulfate 6-0 endosulfatases in intervertebral disc homeostasis. Histol Histopathol 2019;34:1051-60.


110. Lin D, Alberton P, Delgado Caceres M, et al. Loss of tenomodulin expression is a risk factor for age-related intervertebral disc degeneration. Aging Cell 2020;19:e13091.




111. Teeple E, Aslani K, Shalvoy MR, et al. Lubricin deficiency in the murine lumbar intervertebral disc results in elevated torsional apparent modulus. J Biomech 2015;48:2210-3.



112. Dateki S. ACAN mutations as a cause of familial short stature. Clin Pediatr Endocrinol 2017;26:119-25.



113. Tompson SW, Merriman B, Funari VA, et al. A recessive skeletal dysplasia, SEMD aggrecan type, results from a missense mutation affecting the C-type lectin domain of aggrecan. Am J Hum Genet 2009;84:72-9.



114. Anderson IJ, Tsipouras P, Scher C, et al. Spondyloepiphyseal dysplasia, mild autosomal dominant type is not due to primary defects of type II collagen. Am J Med Genet 1990;37:272-6.


115. Eyre S, Roby P, Wolstencroft K, et al. Identification of a locus for a form of spondyloepiphyseal dysplasia on chromosome 15q26.1: exclusion of aggrecan as a candidate gene. J Med Genet 2005;42:e34.



116. Gleghorn L, Ramesar R, Beighton P, et al. A mutation in the variable repeat region of the aggrecan gene (AGC1) causes a form of spondyloepiphyseal dysplasia associated with severe, premature osteoarthritis. Am J Hum Genet 2005;77:484-90.



117. Nilsson O, Guo MH, Dunbar N, et al. Short stature, accelerated bone maturation, and early growth cessation due to heterozygous aggrecan mutations. J Clin Endocrinol Metab 2014;99:E1510-8.



118. Kim SJ, Yoon JS, Hwang IT. A novel heterozygous ACAN variant in a short patient born small for gestational age with recurrent patellar dislocation: a case report. J Clin Res Pediatr Endocrinol 2022;14:481-4.



119. Karatas E, Demir M, Ozcelik F, et al. A case of short stature caused by a mutation in the ACAN gene. Mol Syndromol 2023;14:123-8.




120. Huang H, Jin J, Xiang R, et al. Case report: a novel heterozygous frameshift mutation of ACAN in a Chinese family with short stature and advanced bone age. Front Genet 2023;14:1101695.



121. Stattin EL, Lindblom K, Struglics A, et al. Novel missense ACAN gene variants linked to familial osteochondritis dissecans cluster in the C-terminal globular domain of aggrecan. Sci Rep 2022;12:5215.




122. Fukuhara Y, Cho SY, Miyazaki O, et al. The second report on spondyloepimetaphyseal dysplasia, aggrecan type: a milder phenotype than originally reported. Clin Dysmorphol 2019;28:26-9.



123. Camera G, Stella G, Camera A. New X linked spondyloepimetaphyseal dysplasia: report on eight affected males in the same family. J Med Genet 1994;31:371-6.



124. Meester JA, Vandeweyer G, Pintelon I, et al. Loss-of-function mutations in the X-linked biglycan gene cause a severe syndromic form of thoracic aortic aneurysms and dissections. Genet Med 2017;19:386-95.




125. Cho SY, Bae JS, Kim NKD, et al. BGN mutations in X-linked spondyloepimetaphyseal dysplasia. Am J Hum Genet 2016;98:1243-8.



126. Arikawa-Hirasawa E, Wilcox WR, Le AH, et al. Dyssegmental dysplasia, Silverman-Handmaker type, is caused by functional null mutations of the perlecan gene. Nat Genet 2001;27:431-4.



127. Nicole S, Davoine CS, Topaloglu H, et al. Perlecan, the major proteoglycan of basement membranes, is altered in patients with Schwartz-Jampel syndrome (chondrodystrophic myotonia). Nat Genet 2000;26:480-3.



128. Rieubland C, Jacquemont S, Mittaz L, et al. Phenotypic and molecular characterization of a novel case of dyssegmental dysplasia, Silverman-Handmaker type. Eur J Med Genet 2010;53:294-8.


129. Ladhani NN, Chitayat D, Nezarati MM, et al. Dyssegmental dysplasia, Silverman-Handmaker type: prenatal ultrasound findings and molecular analysis. Prenat Diagn 2013;33:1039-43.



130. Bauché S, Boerio D, Davoine CS, et al. Peripheral nerve hyperexcitability with preterminal nerve and neuromuscular junction remodeling is a hallmark of Schwartz-Jampel syndrome. Neuromuscul Disord 2013;23:998-1009.


131. Lin PY, Hung JH, Hsu CK, et al. A novel pathogenic HSPG2 mutation in Schwartz-Jampel Syndrome. Front Neurol 2021;12:632336.



132. Gruber HE, Ingram JA, Davis DE, et al. Increased cell senescence is associated with decreased cell proliferation in vivo in the degenerating human annulus. Spine J 2009;9:210-5.


133. Roberts S, Evans EH, Kletsas D, et al. Senescence in human intervertebral discs. Eur Spine J 2006;15 Suppl 3:S312-6.




135. Novais EJ, Tran VA, Johnston SN, et al. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat Commun 2021;12:5213.




136. Smith MM, Hayes AJ, Melrose J. Pentosan polysulfate, a semisynthetic heparinoid disease-modifying osteoarthritic drug with roles in intervertebral disc repair biology emulating the stem cell instructive and tissue reparative properties of heparan sulfate. Stem Cells Dev 2022;31:406-30.


137. Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 2015;14:644-58.


138. Hickson LJ, Langhi Prata LGP, Bobart SA, et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019;47:446-56.



139. Xu HW, Fang XY, Liu XW, et al. Alpha-Ketoglutaric acid ameliorates intervertebral disk degeneration by blocking the IL-6/JAK2/STAT3 pathway. Am J Physiol Cell Physiol 2023;325:C1119-30.


- TOOLS
-
METRICS

-
- 1 Crossref
- Scopus
- 663 View
- 55 Download


























