INTRODUCTION
Compromised hand dexterity represents the predominant and early manifestations observed in degenerative cervical myelopathy (DCM), commonly referred to as myelopathy hand [
1-
3]. The 10-second grip and release (10-s G&R) test plays a pivotal role in evaluating dexterity [
3,
4]. Ono et al. [
3] firstly introduced the 10-s G&R test and demonstrated that fewer than 20 G&R cycles within 10 seconds indicate symptomatic signs in DCM. However, the 10-s G&R test, though simple for clinical use, exhibits interobserver variability when counted by naked eyes [
3]. Various assessment modes, such as Leap Motion sensors [
5-
7] and wisegloves integrated with virtual reality [
8], have been developed to detect variations in hand dexterity based on the 10-s G&R test. However, the need for additional specialized equipment is less convenient and accessible for patients, hindering their widespread adoption and suitability for routine monitoring. Recently, the study by Ibara et al. presented a smartphone-based recording system for the 10-s G&R test, demonstrating its effectiveness in accurately screening for DCM. Nevertheless, given the limited statistical power inherent in such a small sample size, comprising only 22 DCM patients and 17 controls [
9], further research will be crucial to firmly establish the clinical utility and determine its generalizability across heterogeneous DCM populations. Currently, a widely accepted, easily accessible, effective, and replicable evaluation method for clinical application is lacking. Recently, our team proposed the 3D-MobileNetV2 model, employing convolutional neural networks, which has achieved 97.4% accuracy in counting G&R cycles [
10]. Significantly, our approach eliminates the need for additional equipment, enhances accessibility, and can be utilized in a variety of settings.
Our primary objective here is to evaluate the diagnostic performance of deep learning-enhanced hand grip and release test (DL-HGRT) in predicting DCM. Furthermore, we aim to explore its capability to reduce the duration of the 10-s G&R test.
DISCUSSION
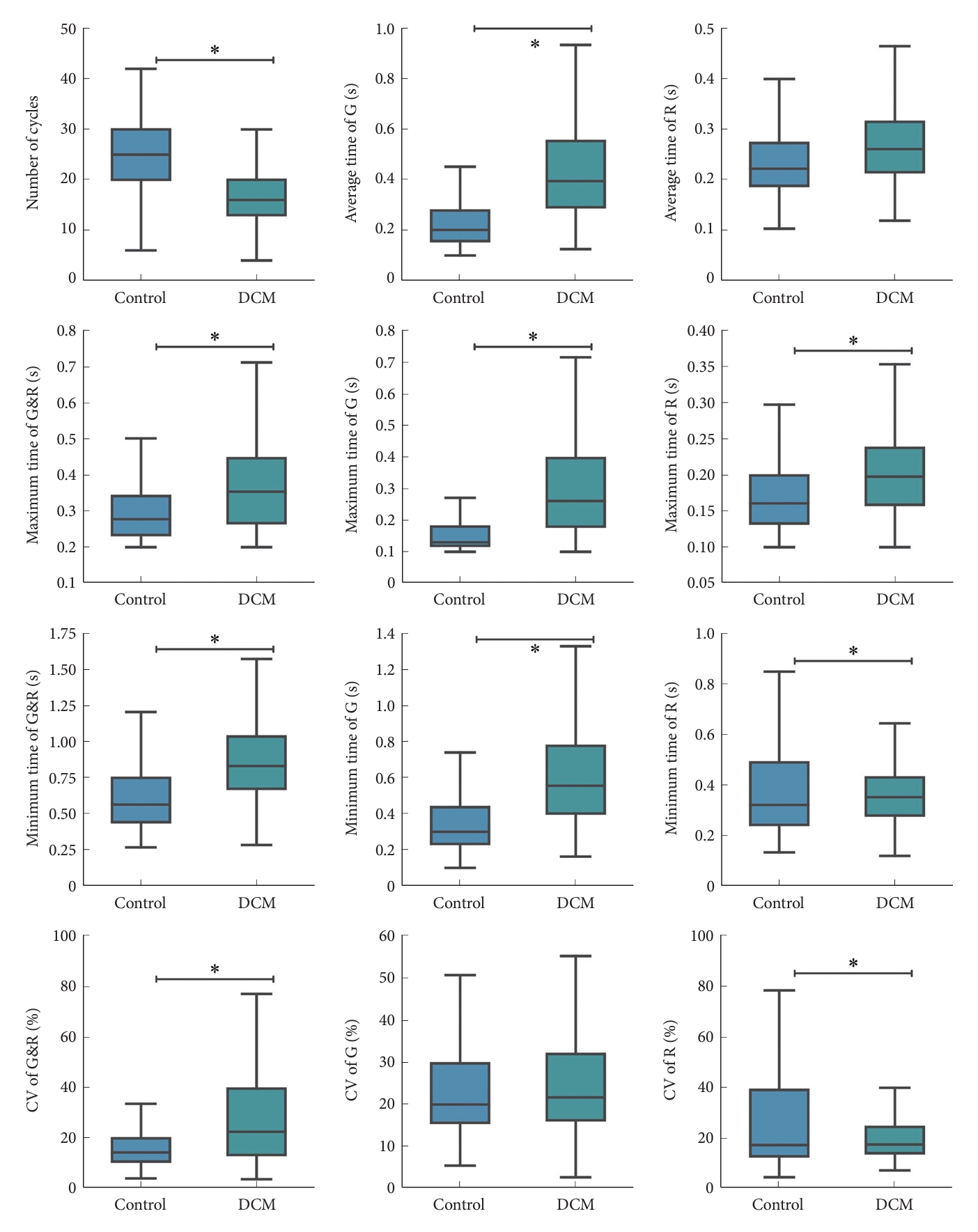
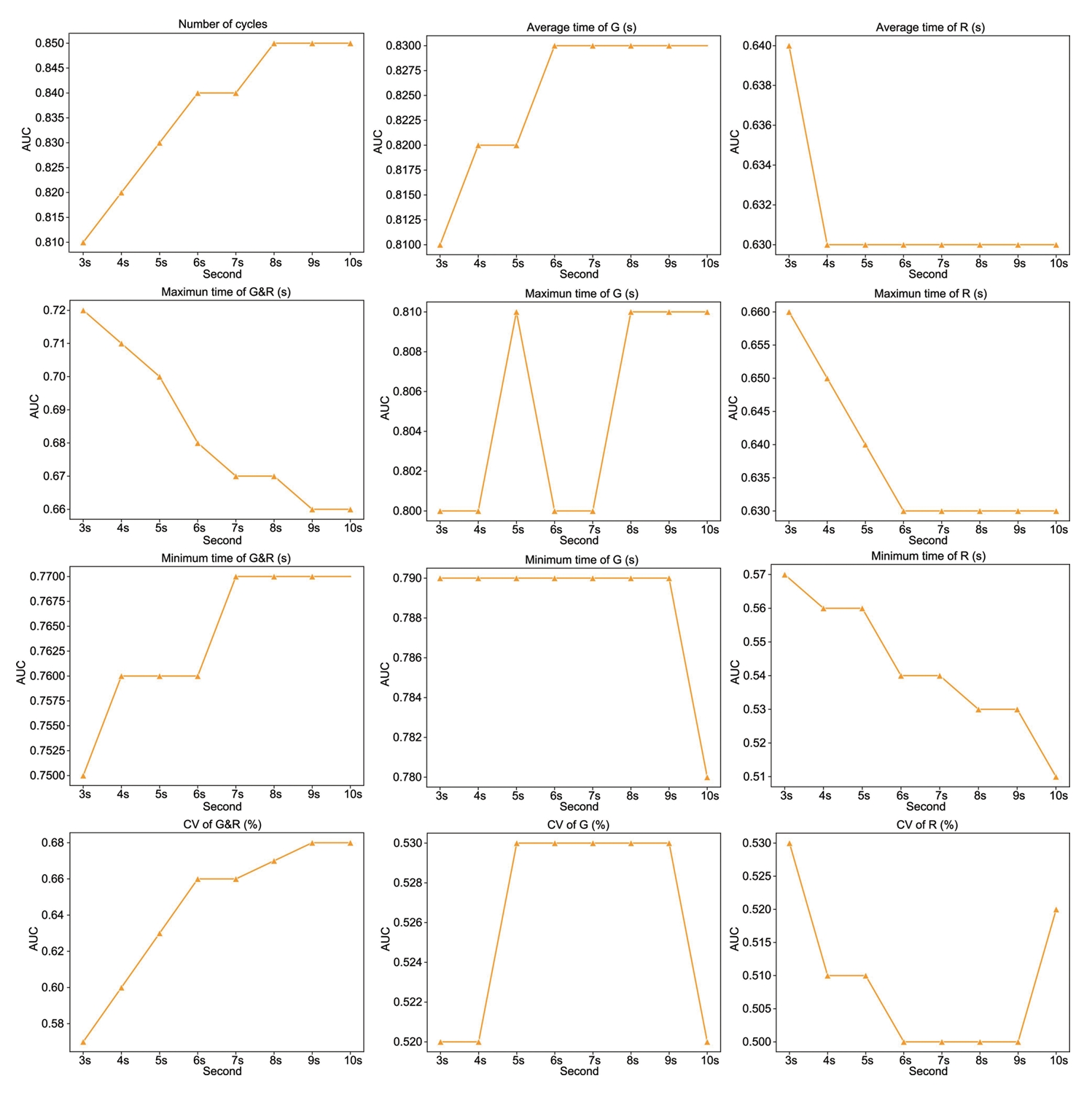
Our investigation demonstrates that the 10-s G&R test, enhanced by our DL-HGRT, effectively distinguishes DCM patients from controls. Notably, the number of cycles and average grip time yield the most robust performance. Furthermore, our findings suggest that the test duration can be shortened to 6 seconds without compromising diagnostic accuracy in terms of average grip time, thus enhancing practicality and user-friendliness. In summary, our results indicate that DL-HGRT offers a practical and user-friendly supplementary tool for accurate DCM assessment, eliminating the need for specialized equipment and indicating potential use in remote or in-home screening, monitoring, and follow-up.
Individuals affected by DCM experience debilitating impairments in upper limb function. Myelopathy hand serves as a valuable marker, offering insights into the functional status of the cervical spinal cord and, concurrently, providing a reflection of the severity of DCM [
3,
14-
16]. Apart from the presence of finger escape sign, an increasing occurrence of uncoordinated finger movements and trick wrist motions was frequently observed in individuals affected by DCM [
14]. Given that hand dysfunction stands as the predominant manifestation of DCM, reseachers have developed numerous approaches to evaluate hand function [
17-
19]. However, the assessment methods employed, such as motion analysis with microreflective markers [
17], prehension tasks [
18], and the GRASSP-M (Graded Redefined Assessment of Strength, Sensibility, and Prehension Version Myelopathy) [
19], all exhibited complexities and requirements for specialized equipment that may hinder their widespread clinical application, underscoring the crucial need for accessible, quantifiable, reproducible, and user-friendly assessment tools to effectively quantify fine motor deficits in DCM.
In 1987, the 10-s G&R test, proposed by Ono et al. [
3], served as a pioneering tool for evaluating hand function in cervical myelopathy, demonstrating that a presentation of symptomatic signs is associated with performing fewer than 20 grip-release cycles within a 10-second timeframe. Significantly, the 10-s G&R test has been demonstrated to be highly reproducible and effective in the assessment of DCM [
4,
11,
18]. Researchers have devoted their attention to enhancing the convenience, reproducibility, and applicability of hand dexterity assessment based on the 10-s G&R test for diagnostic, monitoring, and follow-up purposes. Hosono et al. [
4] validated the G&R test for the assessment of the severity of DCM. However, the reliance on video recording and manual cycle counting introduces potential concerns regarding feasibility and measurement variability. Su et al. [
8] introduced the myelopathy-hand functional evaluation system, which employs virtual reality to quantify hand motor dysfunction based on the 10-s G&R test in DCM patients. Yet its complexity and lack of portability may hinder routine clinical use. In the work by Koyama et al. [
5], their machine learning model, utilizing a Leap Motion sensor, demonstrated reasonably high sensitivity but exhibited lower specificity in the classification of DCM. Nevertheless, the limitations, including a small sample size and the reliance on a specialized sensor, constrained the generalizability of their findings. Ibara et al. present a novel smartphone-based method for DCM screening, demonstrating high diagnostic accuracy, but with limitations, including a small sample size, which calls for further validation in larger and diverse cohorts to establish clinical utility [
9]. Therefore, the limitations observed in the aforementioned studies encompass issues related to feasibility, measurement variability, complexity, lack of portability, restricted sample sizes, and a dependence on specialized sensors or equipment. In our study, we exclusively used the front-facing cameras of smartphones for recording the 10-s G&R test and conducted autonomous processing, indicating great potential in remote or in-home screening and monitoring. Our findings robustly demonstrate that the 10-s G&R test, enhanced by our DL-HGRT, efficiently distinguishes patients with DCM from control subjects.
Regarding the assessment duration of the G&R test, the 10-second interval stands as the most classical and widely employed timeframe in clinical practice [
11,
18]. In a study by Hosono et al. [
4], the assessment duration of the G&R test was extended to 15 seconds to validate its performance against JOA scores and was found to be both reliable and valid, demonstrating its potential for clinical use. However, a compelling insight emerges when contemplating a reduction in the testing duration. Our investigation uncovers that the mean grip time achieves its peak, demonstrating an impressive AUC of 0.83, within the initial 6 seconds. Beyond this point, extending the duration to the conventional 10 seconds does not yield a substantial improvement in diagnostic accuracy. Our evidence suggests that a 6-second testing interval proves to be sufficiently effective for the comprehensive evaluation of hand motor dysfunction in DCM, particularly when utilizing our DL-HGRT. It is crucial to recognize that the traditional 10-second G&R test, when counted by the naked eye, may indeed appear less significantly useful whether counted for 10 or 6 seconds. However, in the era of rapid internet development, the reduction of time by 40% holds significance, particularly in terms of data transmission and storage. In our study, the videos of the 10-s G&R test were transmitted to our remote server, where they were analyzed, and the generated parameters were then sent back to our smartphone. This process is designed for efficient video size reduction and swift transmission. 40% reduction in time becomes even more efficacious and applicable with the integration of artificial intelligence, contributing to the overall feasibility and practicality of our proposed method. Nonetheless, the need for more extensive and forward-looking investigations arises to provide further clarification on the significance of the 6s G&R test in the diagnosis of DCM.
In this study, we have designed DL-HGRT integrated with 3D-MobileNetV2, which exhibits exceptional diagnostic performance in predicting DCM. The number of cycles emerges as the most robust diagnostic indicator, achieving an AUC of 0.85, a sensitivity of 80.12%, and a specificity of 74.29% at the optimal threshold of 20 cycles. A prior investigation demonstrated that the 10-s G&R test, employed for the screening of patients with DCM, exhibited sensitivity ranging from 61% to 77%, specificity from 52% to 66%, and the AUC varied within the range of 0.71 to 0.77 [
11]. Koyama et al. [
5] conducted the analysis of hand movements during the 10-s G&R test for DCM screening. They employed Leap Motion and achieved commendable results, with a sensitivity of 84.0%, specificity of 60.7%, and an AUC of 0.85. In the study conducted by Ibara et al. [
9], a count-based model exhibited a sensitivity of 79.4%, specificity of 86.4%, and an AUC of 0.87. The final proposed model displayed even more impressive results, with a sensitivity of 90.9%, specificity of 88.2%, and an AUC of 0.93. Regarding diagnostic performance, our proposed model is on par with or even surpasses previous studies employing the 10-s G&R test. In summary, our investigation demonstrates robust diagnostic capabilities, making a valuable contribution to clinical practice and holding the potential to enhance DCM diagnosis.
It is important to acknowledge the limitations of the study. First, not all confounding variables were considered in the analysis. Nonetheless, efforts were made to minimize the impact of these factors on hand function. PSM was employed to mitigate potential outcome bias related to age and sex, which are known to influence the 10-s G&R test results [
8,
11,
20,
21]. Second, not everyone in the control group underwent magnetic resonance imaging assessment, raising the possibility that some individuals in the control group might have undiagnosed DCM. However, attempts were made to exclude this possibility through comprehensive history-taking, physical examination, and the assessment of relevant existing images. Third, the study did not include participants with other conditions affecting hand movement, and it remains unclear whether the examination can effectively distinguish DCM from other disorders. Nonetheless, patients with hand disorders resulting from various other conditions, such as amyotrophic lateral sclerosis, Parkinson disease, and cerebrovascular disease, have been recruited for the purpose of differential diagnosis in our future study. Finally, it is important to recognize that this study was conducted at a single center, which may introduce selection bias. Expanding the patient pool across multiple centers in a prospective study in the future would enhance the representativeness of the study.