 |
 |
- Search
|
|
||
Abstract
Objective
Methods
Results
NOTES
Conflict of Interest
The CUVIS-spine robot mentioned in this paper is produced by CUREXO Inc. Seong Yi serves as a paid consultant for CUREXO Inc. Yoon Ha Hwang, Byeong-Jin Ha, Hyung Cheol Kim, Byung Ho Lee, Jeong-Yoon Park, and Dong-Kyu Chin state that they have no commercial or financial associations that might be perceived as a potential conflict of interest.
Funding/Support
This research received funding from CUREXO Inc., Republic of Korea (Grant/award number: 2017-31-1035, 2019-31-0831); Korea Institute for Robot Industry Advancement; and Domestic Medical Device Training Support Center, Ministry of Health and Welfare, and Korea Health Industry Development Institute.
Fig. 1.
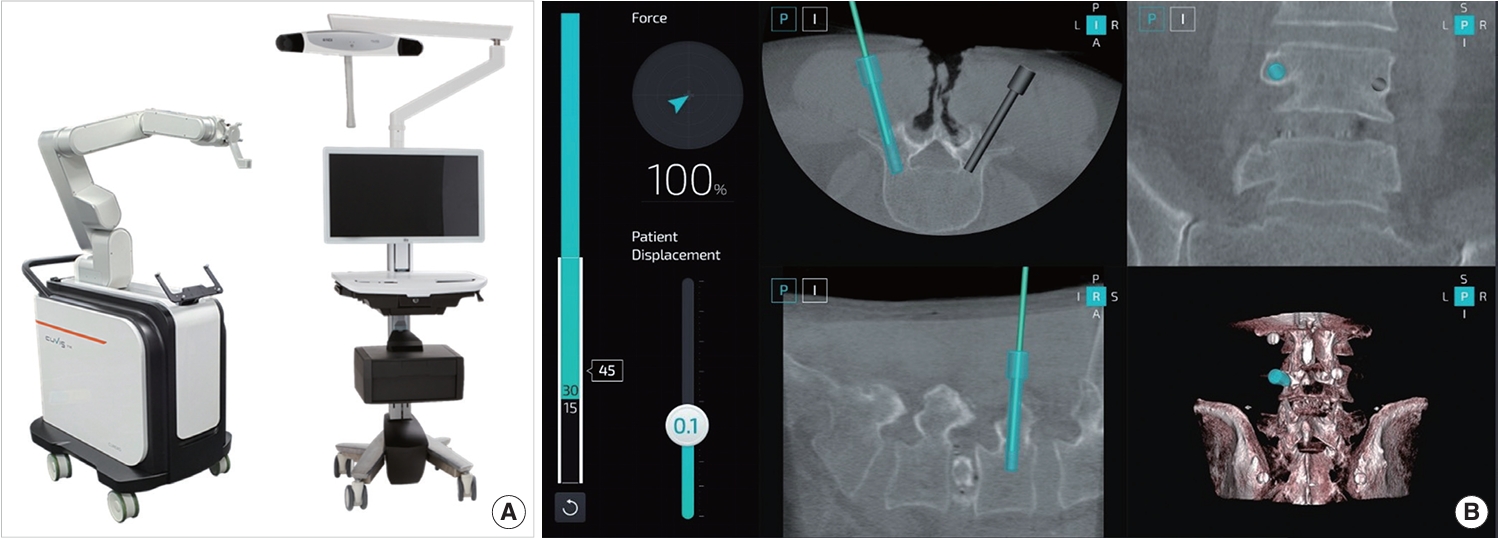
Fig. 2.
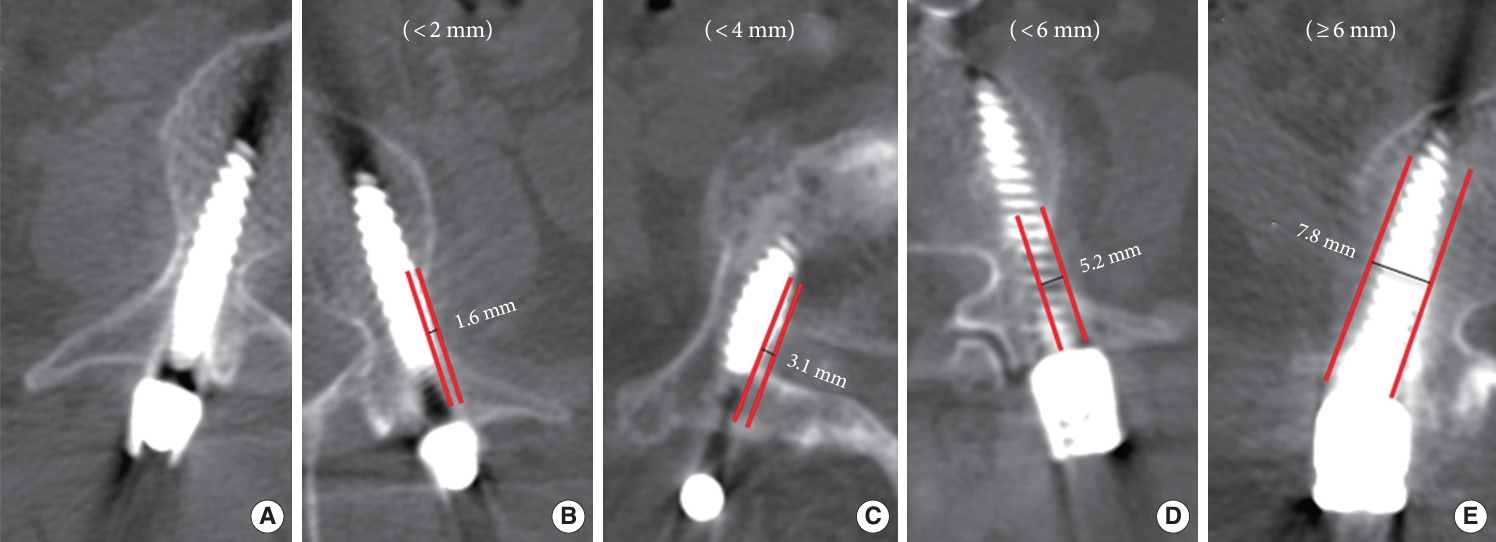
Fig. 3.
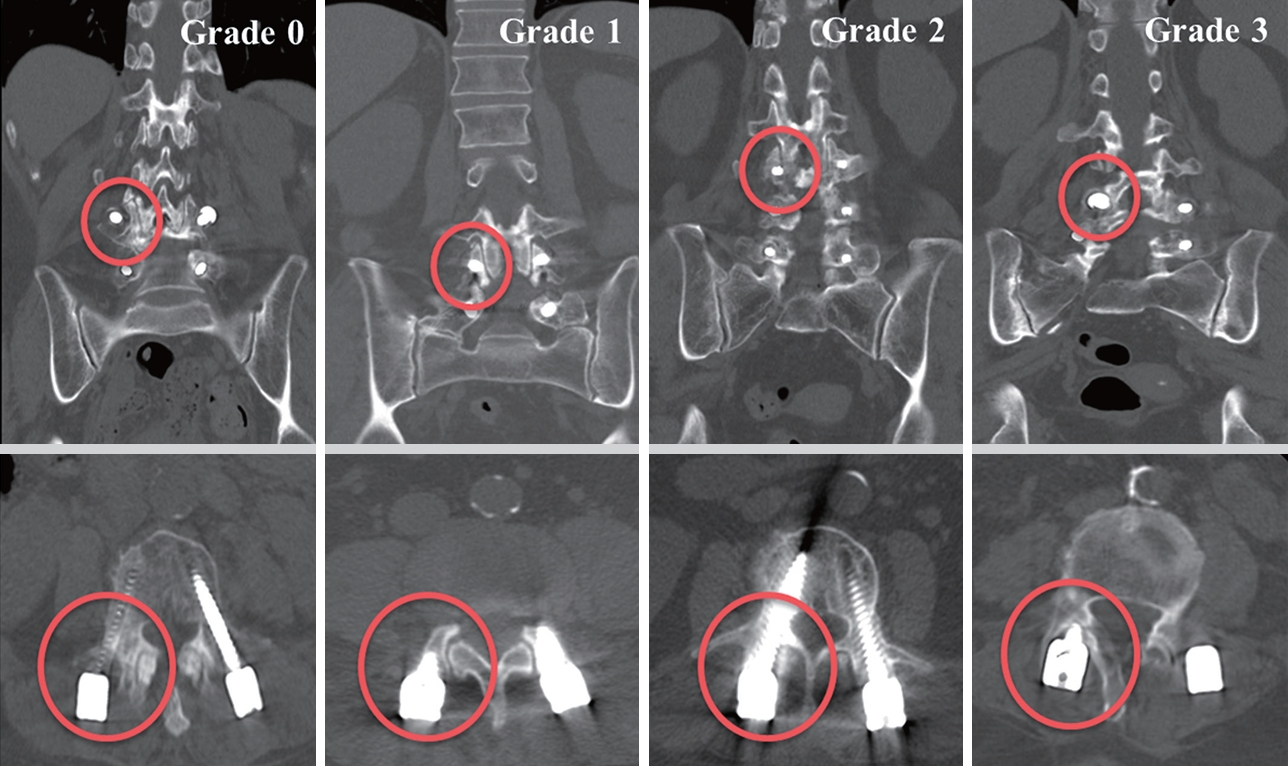
Fig. 4.

Table 1.
| Characteristic | RS (N = 54) | CG (N = 54) | FG (N = 54) | p-value* |
|---|---|---|---|---|
| Demographic | ||||
| Sex | 0.883 | |||
| Male | 16 (29.6) | 14 (25.9) | 14 (25.9) | |
| Female | 38 (70.4) | 40 (74.1) | 40 (74.1) | |
| Age (yr) | 68.5 ± 7.2 | 68.0 ± 6.6 | 65.5 ± 6.8 | 0.057 |
| BMI (kg/m2) | 24.1 ± 3.0 | 24.7 ± 2.1 | 24.6 ± 2.9 | 0.402 |
| BMD | -1.6 ± 1.2 | -1.7 ± 1.4 | -1.6 ± 0.8 | 0.946 |
| Baseline PROMs | ||||
| VAS for back pain | 5.7 ± 2.5 | 5.9 ± 2.0 | 5.7 ± 2.1 | 0.916 |
| VAS for leg pain | 7.0 ± 2.5 | 6.9 ± 2.0 | 7.0 ± 2.2 | 0.948 |
| ODI | 44.4 ± 14.0 | 45.6 ± 17.0 | 45.9 ± 17.3 | 0.881 |
| SF-36 PCS | 35.4 ± 14.7 | 36.1 ± 16.0 | 35.9 ± 14.2 | 0.969 |
| SF-36 MCS | 54.5 ± 17.2 | 55.4 ± 15.4 | 53.5 ± 17.8 | 0.834 |
| Operation level | 0.973 | |||
| L2/L3 | 3 (5.6) | 2 (3.7) | 2 (3.7) | |
| L3/L4 | 10 (18.5) | 8 (14.8) | 11 (20.4) | |
| L4/L5 | 38 (70.4) | 42 (77.8) | 39 (72.2) | |
| L5/S1 | 3 (5.6) | 2 (3.7) | 2 (3.7) | |
| Preoperative spinal condition | ||||
| Spinal alignment | ||||
| PI (°) | 25.7 ± 7.5 | 26.6 ± 7.3 | 25.6 ± 7.1 | 0.749 |
| LL (°) | 30.4 ± 10.7 | 30.7 ± 11.1 | 32.2 ± 10.4 | 0.648 |
| PT (°) | 56.1 ± 13.0 | 57.3 ± 12.7 | 57.8 ± 12.5 | 0.772 |
| SS (°) | 40.1 ± 13.0 | 41.3 ± 12.7 | 41.8 ± 12.5 | 0.772 |
| Pedicle diameter | ||||
| Width (mm) | 13.9 ± 2.5 | 13.7 ± 2.4 | 13.9 ± 2.2 | 0.858 |
| Height (mm) | 14.5 ± 1.5 | 14.5 ± 1.6 | 14.3 ± 1.5 | 0.646 |
| Adjacent degeneration | ||||
| Disc degeneration | 2.5 ± 0.7 | 2.6 ± 0.9 | 2.6 ± 0.8 | 0.468 |
| Facet degeneration | 1.3 ± 0.8 | 1.2 ± 0.8 | 1.3 ± 0.7 | 0.902 |
| Surgical related outcomes | ||||
| Hospital stay (day) | 8.5 ± 1.6 | 10.4 ± 2.3† | 10.1 ± 2.4‡ | < 0.001 |
| Operation minute | 243.3 ± 43.6 | 103.0 ± 24.9† | 155.3 ± 41.9‡,§ | < 0.001 |
| Complications | ||||
| Wound infection | 0 (0) | 1 (1.9) | 3 (5.9) | 0.166 |
| Root injury | 1 (1.9) | 1 (1.9) | 0 (0) | 0.603 |
| Pedicle fracture | 0 (0) | 1 (1.9) | 1 (1.9) | 0.603 |
Values are presented as number (%) or mean±standard deviation.
RS, robotic surgery; CG, C-arm guidance; FG, free of guidance; BMI, body mass index; BMD, bone mineral density; PROMs, patient-reported outcome measures; VAS, visual analogue scale; ODI, Oswestry disability index; SF-36, 36-item Short Form health survey; PCS, physical composite score; MCS, mental composite score; PI, pelvic incidence; LL, lumbar lordosis; PT, pelvic tilt; SS, sacral slope.
Higher disc and facet degeneration indicated severe preoperative degenerative condition.
All the above group comparisons were confirmed by a post hoc test.
Table 2.
| Variable | RS (N = 216) | CG (N = 216) | FG (N = 216) | p-value* |
|---|---|---|---|---|
| Gertzbein-Robbins scale (GRS) | < 0.001 | |||
| A | 199 (92.1) | 156 (72.2) | 181 (83.8) | |
| B | 14 (6.5) | 44 (20.4) | 26 (12.0) | |
| A+B | 213 (98.6) | 200 (92.6) | 207 (95.8) | |
| C | 2 (0.9) | 10 (4.6) | 8 (3.7) | |
| D | 1 (0.5) | 2 (0.9) | 1 (0.5) | |
| E | 0 (0) | 4 (1.9) | 0 (0) | |
| Total | 19.6 ± 0.8 | 18.4 ± 1.8† | 19.2 ± 1.1§ | < 0.001 |
| RS (N = 108) | CG (N = 108) | FG (N = 108) | p-value* | |
| Babu classification (proximal facet joints violation) | 0.002 | |||
| 0 | 91 (84.3) | 84 (77.8) | 68 (63.0) | |
| 1 | 13 (12.0) | 18 (16.7) | 29 (26.9) | |
| 2 | 3 (2.8) | 6 (5.6) | 9 (8.3) | |
| 3 | 1 (0.9) | 0 (0) | 2 (1.9) | |
| Total | 7.6 ± 0.9 | 7.4 ± 0.9 | 7.0 ± 1.1‡,§ | < 0.001 |
Values are presented as mean±standard deviation or number (%).
RS, robotic surgery; CG, C-arm guidance; FG, free of guidance.
Total, sum of scores assigned to each graded screws within a single patient.
GRS: 5 points for grade A, 4 points for grade B, 3 points for grade C, 2 points for grade D, and 1 point for grade E (ranging from 4–20).
Babu: 4 points for grade 0, 3 points for grade 1, 2 points for grade 2, and 1 point for grade 3 (ranging from 2–8).
Higher GRS and Babu total scores indicated higher accuracy levels.
All the above group comparisons were confirmed by a post hoc test.
Table 3.
| Deviation |
No. of screws |
||
|---|---|---|---|
| RS | CG | FG | |
| Cranial | 0 (0) | 4 (1) | 0 (0) |
| Caudal | 0 (0) | 0 (0) | 0 (0) |
| Medial | 5 (0) | 6 (0) | 16 (2) |
| Lateral | 12 (3) | 50 (15) | 19 (7) |
| Total | 17 (3) | 60 (16) | 35 (9) |
Table 4.
| Variable | RS (N = 54) | CG (N = 54) | FG (N = 54) | p-value* |
|---|---|---|---|---|
| Radiological outcome | ||||
| Weiner classification | 0.326 | |||
| 0 | 35 (64.8) | 24 (44.4) | 27 (50.0) | |
| 1 | 9 (16.7) | 18 (33.3) | 13 (24.1) | |
| 2 | 7 (13.0) | 6 (11.1) | 9 (16.7) | |
| 3 | 3 (5.6) | 6 (11.1) | 5 (9.3) | |
| Radiological ASD† | 0.651 | |||
| None | 44 (81.5) | 42 (77.8) | 40 (74.1) | |
| Failure | 10 (18.5) | 12 (22.2) | 14 (25.9) | |
| Mechanical failure‡ | 0.788 | |||
| None | 49 (90.7) | 47 (87.0) | 47 (87.0) | |
| Failure | 5 (9.3) | 7 (13.0) | 7 (13.0) | |
| Clinical outcome | ||||
| Post operative PROMs | ||||
| VAS back | 3.4 ± 2.2 | 3.1 ± 1.3 | 3.6 ± 1.6 | 0.258 |
| VAS leg | 3.6 ± 2.8 | 3.2 ± 1.3 | 3.3 ± 1.7 | 0.535 |
| ODI | 28.1 ± 15.8 | 24.6 ± 15.1 | 24.2 ± 12.4 | 0.366 |
| SF-36 PCS | 51.8 ± 20.7 | 59.4 ± 15.5 | 54.2 ± 14.9 | 0.078 |
| SF-36 MCS | 60.6 ± 19.8 | 58.2 ± 13.1 | 58.9 ± 16.9 | 0.760 |
| Clinical ASD | 0.899 | |||
| None | 51 (94.4) | 51 (94.4) | 50 (92.6) | |
| Failure | 3 (5.6) | 3 (5.6) | 4 (7.4) |
Values are presented as number (%) or mean±standard deviation.
RS, robotic surgery; CG, C-arm guidance; FG, free of guidance; ASD, adjacent segmental disease; PROMs, patient-reported outcome measures; VAS, visual analogue scale; ODI, Oswestry disability index; SF-36, 36-item Short Form health survey; PCS, physical composite score; MCS, mental composite score.
Table 5.
REFERENCES

-
METRICS

-
- 2 Crossref
- Scopus
- 663 View
- 52 Download


























