 |
 |
- Search
|
|
||
Abstract
Chiari I malformation is characterized by the downward displacement of cerebellar tonsils through the foramen magnum. While discussing the treatment options for Chiari I malformation, the points of focus include: (1) Has the well-established procedure of posterior fossa decompression become outdated and has been replaced by posterior C1–2 stabilization in every case? (2) In case posterior stabilization is required, should a C1–2 stabilization, rather than an occipitocervical fusion, be the only procedure recommended? The review of literature revealed that when there is bony instability like atlantoaxial dislocation (AAD), occipito-atlanto-axial facet joint asymmetry or basilar invagination (BI) associated with Chiari I malformation, one should address the anterior bony compression as well as perform stabilization. This takes care of the compromised canal at the foramen magnum and re-establishes the cerebrospinal fluid flow along the craniospinal axis; and also provides treatment for CVJ instability. In the cases with a pure Chiari I malformation without AAD or BI and with completely symmetrical C1–2 joints, however, posterior fossa decompression with or without duroplasty is sufficient to bring about neurological improvement. The latter subset of cases with pure Chiari I malformation have, thus, shown significant (>70%) rates of neurological improvement with posterior fossa decompression alone. A C1–2 posterior stabilization is a more stable construct due to the strong bony purchase provided by the C1–2 lateral masses and the short lever arm of the construct. However, in the cases with significant bleeding from paravertebral venous plexus; a very high BI, condylar hypoplasia and occipitalized atlas; gross C1–2 rotation or vertical C1–2 joints with unilateral C1 or C2 facet hypoplasia, as well as the presence of subaxial scoliosis; maldevelopment of the lateral masses and facet joints (as in very young patients); or, the artery lying just posterior to the C1–2 facet joint capsule (being endangered by the C1–2 stabilization procedure), it may be safer to perform an occipitocervical rather than a C1–2 fusion.
‘Overkill ’ (noun) is defined by the Webster’s dictionary as “an excess of something (such as a quantity or an action) beyond what is required or suitable for a particular purpose.” There is no better example of this word than in the procedures advocated for the management of Chiari I malformation.
Chiari I malformation is defined as a congenital or sporadic abnormality characterized by the downward displacement of cerebellar tonsils through the foramen magnum [1]. It eventually results in a craniospinal dissociation of cerebrospinal fluid (CSF) flow caused by selective obstruction at the foramen magnum and leads to cerebellar and bulbar dysfunction, and associated conditions such as syringomyelia and hydrocephalus. It may often be associated with other bony craniocervical junction (CVJ) anomalies, such as atlantoaxial dislocation (AAD), basilar invagination (BI), rotatory C1–2 dislocation and asymmetrical facet joints.
We have come a long way from Hans Chiari’s original definition of the hindbrain malformation in 1891 [2], and the evolving knowledge related to its pathophysiology has transformed its management over the years, being influenced by the works of Gardner [3], Gardner and McMurray [4], Williams [5,6], Aboulker [7], Oldfield et al. [8], and Oldfield [9] What has not changed is the fact that the epicenter of the problem lies at the CVJ, and thus, most of the treatment strategies are directed here. The traditional and established method of treating Chiari I malformation has been by utilizing a posterior fossa decompression with or without duroplasty; the standard posterior fossa decompression combined with its augmentation by thinning the occipital planum; and, a CSF diversion procedure for the associated hydrocephalus. Recently, there have been reports of cervical fusion (C1–2) being utilized as a management strategy for Chiari malformation on the premise that CVJ instability is responsible for the descent of tonsil through the posterior fossa. The rationale is that C1–2 stabilization effectively prevents further cervicomedullary damage brought about by the persistent C1–2 instability, that is aggravated by flexion-extension movements of the neck [10].
The 2 questions that immediately come to mind while discussing the treatment options for Chiari I malformation are:
(1) Has the well-established procedure of posterior fossa decompression become outdated and is now no longer indicated? Instead, should a posterior C1–2 stabilization be performed in all cases?
(2) In case of stabilization is required, should C1–2 stabilization procedure, rather than an occipitocervical fusion, be the only procedure recommended?
This review attempts to analyze and answer these questions.
Rather than adopting an Orwellian (totalitarian) view that ‘the only way is one way that is my way!’, let us scientifically examine literature on whether or not the procedure of posterior fossa decompression has actually been effective at all, on a long-term basis, in patients with Chiari I malformation. We reviewed all available literature by doing a PubMed search with the keywords: “Chiari malformation,” “Posterior fossa decompression,” “Chiari-I malformation,” and “Foramen magnum decompression” and included all studies with more than 10 adult patients, that had reported long-term outcomes (Table 1) [3,11-27].
A perusal of this review shows that posterior fossa decompression has been largely effective with most of the recent studies showing a >70% improvement rate on a long-term basis in patients with Chiari I malformation. However, it is also evident that 20% to 30% of patients do not attain a significant neurological improvement. This is mainly related to those patients who have a significant cervicomedullary distortion with a small posterior fossa; those with associated significant CVJ anomalies; those with an initial significantly compromised neurological status that did not show any improvement following posterior fossa decompression; and, especially those with persistent syringomyelia. There are, therefore, lacunae in our knowledge regarding the management of this entity; however, nearly 70% of the patients do improve after posterior fossa decompression, thereby pointing to the effectiveness of the procedure in a vast majority of cases.
In the largest meta-analysis to date of posterior fossa decompression for Chiari I malformation, Chai et al. [28], compared posterior fossa decompression with or without duroplasty. Fourteen cohort studies comprising 3,666 patients with Chiari malformation type I were analyzed. There was a significant improvement in syringomyelia in both the groups. The decrease in syringomyelia was better in patients in the posterior fossa decompression group with duroplasty than in patients in the posterior fossa decompression group alone (relative risk=1.57; 95% confidence interval, 1.07–2.32; Pheterogeneity=0.042, I2=56.6%). This meta-analysis clearly focuses on the effectiveness of the procedure in a large group of patients reported in literature who underwent posterior fossa decompression/augmentation. Thus, this procedure has been utilized uniformly for the management of Chiari I malformation. An important aspect of its universal appeal is its safety profile, as can be seen from the <1% mortality rates that are reported in most series (Table 1).
There is objective evidence also, based upon CSF dynamic studies at the foramen magnum, of the efficacy of this procedure. In an attempt to determine the CSF flow dynamics at the CVJ in patients with Chiari I malformation, we performed radionuclide cisternography (using technetium diethylene-triamine-pentaacetate [Tc99m-DTPA]) whose flow along the lumbar and cerebral subarachnoid spaces across the foramen magnum was followed over 24 hours. The clinical outcome following the posterior fossa decompression was also assessed in the 17 patients recruited [29]. We were surprised by our results. As expected, we found that posterior decompression and duroplasty provided maximum clinical relief in patients with a demonstrable foramen magnum block on radionuclide cisternography [29]. Even in the patients where there was normal CSF flow or just subarachnoid delay of the contrast at the CVJ (although the foramen magnum diameter was normal), a significant clinical benefit was obtained from posterior fossa decompression. This benefit, like in cases of complete CSF block, was sustained even after 6 months of surgery, that was also evident by the resolution in syringomyelia in most of the cases. The improvement in neurological status following posterior fossa decompression even in patients with partial CSF block or no block at the CVJ can only be explained by Oldfield’s theory of pulsatile aggravation of tonsillar herniation with every cardiac systole that leads to intermittent CSF pathway block at the foramen magnum and a subsequent persistence and aggravation of syringomyelia [9]. This intermittent tonsillar herniation and CSF pathway blockage at the foramen magnum improves after posterior fossa decompression. It also gives credence to the outcomes determined in our patients, as those with a complete foramen magnum block to CSF egress had a better improvement in their neurological status than those with a dynamic block (in patients with a normal contrast flow or a subarachnoid delay of flow). In a recent study focusing on the pathogenesis of Chiari I malformation and the pathophysiology of syringomyelia [9], the Queckenstedt’s test showed either a partial or no increase in intracranial CSF pressure before posterior fossa decompression, but the same was positive after the surgery was over. This means that a communication had been established between the cranial and spinal subarachnoid space following the posterior fossa decompression in patients with Chiari I malformation. There was also a significant improvement in craniospinal compliance after the posterior fossa decompression, though not completely normalizing when compared to controls. The syrinx diameter, syringomyelia pressure, and the cervical subarachnoid pressure corresponded to the systole and respiration cycles on cine magnetic resonance imaging of the CVJ, again proving that Oldfield’s theory of pulsatile tonsillar herniation corresponding to systole held in determining the pathogenesis of clinical manifestations and syringomyelia in patients with Chiari I malformation. This indicates that the posterior fossa decompression has a definite role in normalizing craniospinal pressure gradient in patients with Chiari I malformation.
Our study in 2007 focusing on AAD (with or without BI) with Chiari I malformation, however, revealed contradictory results [30].
We studied a series of 39 cases of Chiari I with AAD—28 patients with an irreducible AAD and 11 with a reducible AAD. The mean foramen magnum diameter did not vary between the two groups: 18.4±0.5 mm in the reducible AAD group and 18.1±0.35 mm in the irreducible AAD group. In 11 patients with a reducible AAD, a direct posterior decompression with duroplasty and fusion was done. In the 28 patients with an irreducible AAD, 18 cases underwent a transoral decompression with posterior stabilization (12 with posterior fossa decompression) and 10 cases underwent a posterior fossa decompression alone (n=3) or with stabilization (n=7). On follow-up evaluation, using the Arora’s modified Klekamp and Samii [31] score used in the study, the 2 groups has a nearly a similar mean score of neurological improvement. The reducible AAD group had a score of 26.18±0.98 while the irreducible group had a score of 26.19±1.52. Thus, both groups significantly improved over their preoperative score (p=0.000). An interesting observation was the fact that a subset of 10 patients (including those operated at other centers) existed having Chiari I malformation with irreducible AAD, who had undergone only a posterior decompression with or without posterior stabilization in the first stage (because either the coexisting AAD had been missed or not been focused upon during the initial management). Seven of them showed significant postoperative deterioration (p=0.000). In these patients, transoral decompression was carried out in the second stage (along with the posterior stabilization in those patients who had not undergone this procedure in the first stage). Following transoral decompression, their mean scores improved significantly so that at the final follow-up, it matched the scores of those who had undergone a single-stage transoral decompression and posterior stabilization. Two important conclusions could be drawn from this study: (1) In patients in bony instability, such as AAD or BI associated with Chiari I malformation (who also often have asymmetrical facet joints and torticollis), addressing the anterior compression at the cervicomedullary junction (rather than the posteriorly existing Chiari I malformation), utilizing either the transoral odontoidectomy (that results in excision of the posteriorly directed odontoid and C2 body in irreducible AAD), or the posterior distraction and stabilization procedure (that results in the change of curvature of a posteriorly directed odontoid to a more vertical one and relieves compression on the anterior cervicomedullary junction in both irreducible and reducible AAD) leads to significant neurological improvement; (2) In these patents, performing a pure posterior decompression aggravates the instability and may lead to a subsequent neurological deterioration. In this subset of patients, a posterior stabilization procedure is mandatory to address the stability.
Taking all of the above findings into perspective, we can safely ascertain certain facts. When there is a bony instability like AAD, occipito-atlanto-axial facet joint asymmetry or BI (the latter is considered as a central dislocation) associated with Chiari I malformation, one should address the anterior bony anomaly. Thus, a C1–2 distraction with stabilization (with or without transoral decompression) to address the anterior bony anomaly is mandated [32]. Posterior fossa decompression/augmentation may not be required in these situations to address the concomitantly existing small posterior fossa or Chiari I malformation, as the procedure directed towards the anteriorly existing bony anomaly takes care of the compromised canal at the foramen magnum and re-establishes the CSF flow along the craniospinal axis across the foramen magnum (Figs. 1, 2) [33]. In the cases with a pure Chiari I malformation without AAD or BI, and with completely symmetrical C1–2 joints, however, posterior fossa decompression with or without duroplasty is sufficient to bring about neurological improvement (Figs. 3, 4). The latter subset of cases with pure Chiari I malformation have, thus, show significant (>70%) rates of neurological improvement with posterior fossa decompression alone, as has been reviewed in Table 1.
We now discuss the second question: Is C1–2 distraction and fusion ‘the only’ procedure recommended for these cases? It is an indisputable fact that, where ever possible, a C1–2 stabilization is preferable to an occipito-cervical fusion, as it is biomechanically more stable in comparison to the latter procedure. Including the occipital bone in the stabilization has its disadvantages. In the cases where there is no occipitalization of atlas, this fusion compromises a part of the neck movements at the occipito-C1–2 joints. The occipital squama and the occipital bone are thin and asymmetrical, and apart from the internal occipital crest, may not have enough thickness to permit a stable plate fixation. The long lever arm of the occipital-cervical fusion has the potential to become biomechanically unstable [34]. The long segment fusion extends from the occiput to at least the C2–4 levels, which restricts neck movements at more levels than is actually required. This also places more strain at the joint spaces at a lower level below the fusion, which may result in the manifestations of adjacent segment disease, a potential instability, or a cervical curvature compromise at a subaxial level. Performance of an occipitocervical fusion often prevents the inclusion of the atlas in the posterior fusion. This is due to the lordotic curvature of the cervical spine, which places the posterior arch of atlas and its lateral masses at a great depth. Thus, any screws placed in the lateral mass of the atlas often cannot be included in the occiptocervical construct. At radiology, therefore, in these cases, despite the stable fixation, the anterior arch of atlas may remain in a dislocated position relative to the axis vertebra. Thus, it is indisputable that a C1–2 distraction and stabilization is the preferred option at the CVJ whenever a bony anomaly is encountered (Fig. 2).
However, a procedure is regarded as being an ‘established’ one, when it brings about a neurological improvement in the majority of cases, is technically simple enough to be replicated by every reasonably experienced surgeon, is suitable for nearly every variation arising in that area, and has a low complication rate. In the presence of significant bleeding from the paravertebral venous plexus; a very high BI, condylar hypoplasia and occipitalized atlas, where the occipital condyle and lateral mass of atlas are fused on either side, so that access to the lateral mass of C1 has to be negotiated at a very high level; gross C1–2 rotation or vertical C1–2 joints with unilateral C1 or C2 facet hypoplasia, as well as in the presence of subaxial scoliosis, where insertion of C1–2 screws may endanger the neuraxis or the ipsilateral vertebral artery; in cases of small children, especially in those with syndromic AAD with Chiari I malformation, where maldevelopment of the lateral masses and facet joints coexist; or, in cases of variation in the course of a vertebral artery (such as its skirting the foramen transversarium of atlas, a low-lying posterior inferior cerebellar artery, a bifid vertebral artery or a persistent first intersegmental artery), so that it lies just posterior to the C1–2 facet joint capsule and is in danger of being injured during the passage of C1–2 screws (Fig. 5), it may be safer to perform an occipito-cervical rather than a C1–2 fusion (Fig. 1). In a patient with an occipitalized atlas, the C1–2 movements are already compromised and the patient adjusts to the restriction of the neck movements either by a subaxial vertebral rotation or by compensatory torso rotation. Thus, he/she is not unduly hampered in neck movements by the inclusion of the additional cervical vertebrae in the construct, as occurs in the occipitocervical fusion. Whether an occipitocervical or a C1–2 fusion is performed, after three months, the stability will be provided not by the construct alone but by the concomitant and adequate bony fusion brought about by decortication of the vertebral surfaces, and by the placement of adequate onlay and strut bone grafts in the region as well as within and around the C1–2 facet joints [35-41].
An important concept that is often neglected in the management of AAD with Chiari I malformation is the role of ‘in situ’ C1–2 or occipito-cervical fusion. In the presence of a very high BI, posterior C1–2 bony overdistraction to reduce the central dislocation, may result in neurological deterioration due to a disproportionate distraction of the spinal cord that remains tethered to the dura by denticulate ligaments (Fig. 6). In the presence of significant rotatory C1–2 dislocation, an attempt to correct the relative rotation of the vertebral bodies to their physiological position may result in torsion of the cervical cord, again leading to the aggravation of myelopathy. The lack of reduction of AAD due to the asymmetrical facet joints may also result in persistent canal compromise (Fig. 7). In all these situations, rather than aiming for a radiologically perfect correction of the C1–2 vertebrae, it is more appropriate to perform either an ‘in situ’ stabilization or perform a slight undercorrection. This prevents the underlying cord from getting manipulated too much.
We conclude with the following insights (Table 2). The patients with pure Chiari I malformation with foramen magnum compromise with symmetrical C1–2 joints and without the presence of an occipito-C1–2 instability, require a posterior fossa duroplasty/augmentation (akin to a lumbar laminectomy/laminoplasty procedure being performed for primary lumbar canal stenosis without any lumbar instability or disc prolapse). In the cases with Chiari I malformation with instability in the form of AAD/BI/torticollis/asymmetrical joints, either a C1–2 fusion with distraction, or an occipito-C2, C3 fusion (with or without transoral decompression) may be performed (akin to a lumbar reduction and posterior lumbar transpedicular and/or interbody fusion procedure being performed in the cases of lumbar spondylolisthesis with lumbar canal compromise).
It would be worthy to note that these are recommendations based on current evidence and advocates a treatment protocol that can be adapted safely and effectively by most centers around the world.
Fig. 1.
(A) T2-weighted sagittal magnetic resonance image of the spine showing Chiari I malformation with syringomyelia. (B) Coronal computed tomography (CT) image showing grossly asymmetrical facet joints. (C) Midsagittal CT scan showing atlantoaxial dislocation with spinal canal compromise. (D) As there was gross asymmetry of the facet joints, C1–2 distraction with occipitocervical posterior fusion was performed after removal of the C1 posterior arch. Care was taken not to perform a complete physiological correction of the torticollis as that may have led to an unacceptable torsion on the spinal cord.
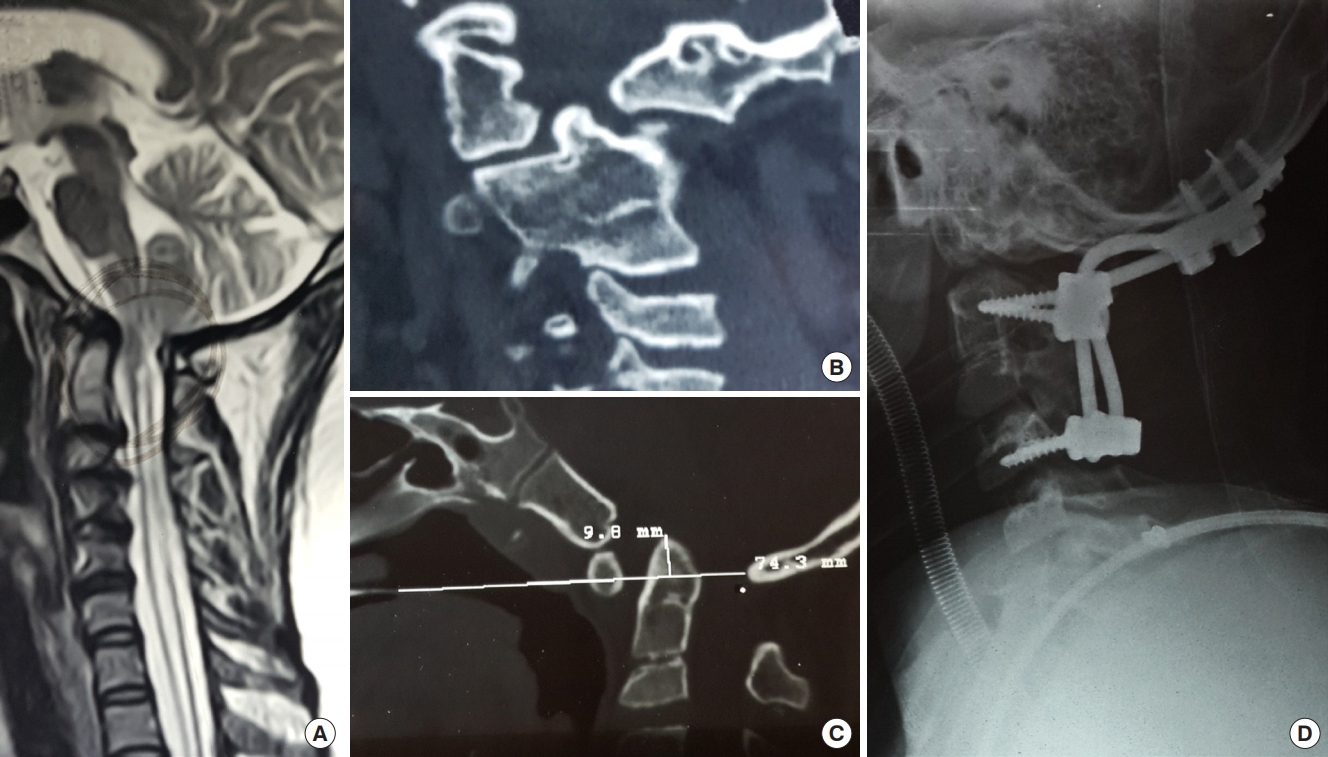
Fig. 2.
(A) Computed tomography (CT) sagittal reconstructed image showing atlantoaxial dislocation (AAD) with mild basilar invagination with occipitalized atlas. (B) Magnetic resonance imaging sagittal T2-weighted image of the cervical spine showing AAD with Chiari I malformation with mild syringomyelia. (C) Three-dimensional coronal reconstructed image showing gross asymmetry of facet joints as well as unilateral vertebral artery hypoplasia. (D) Postoperative sagittal reconstructed CT scan images showing C1–2 stabilization with posterior decompression with reduction of AAD. (E) Coronal CT scan images showing the C1–2 lateral mass screws in place.
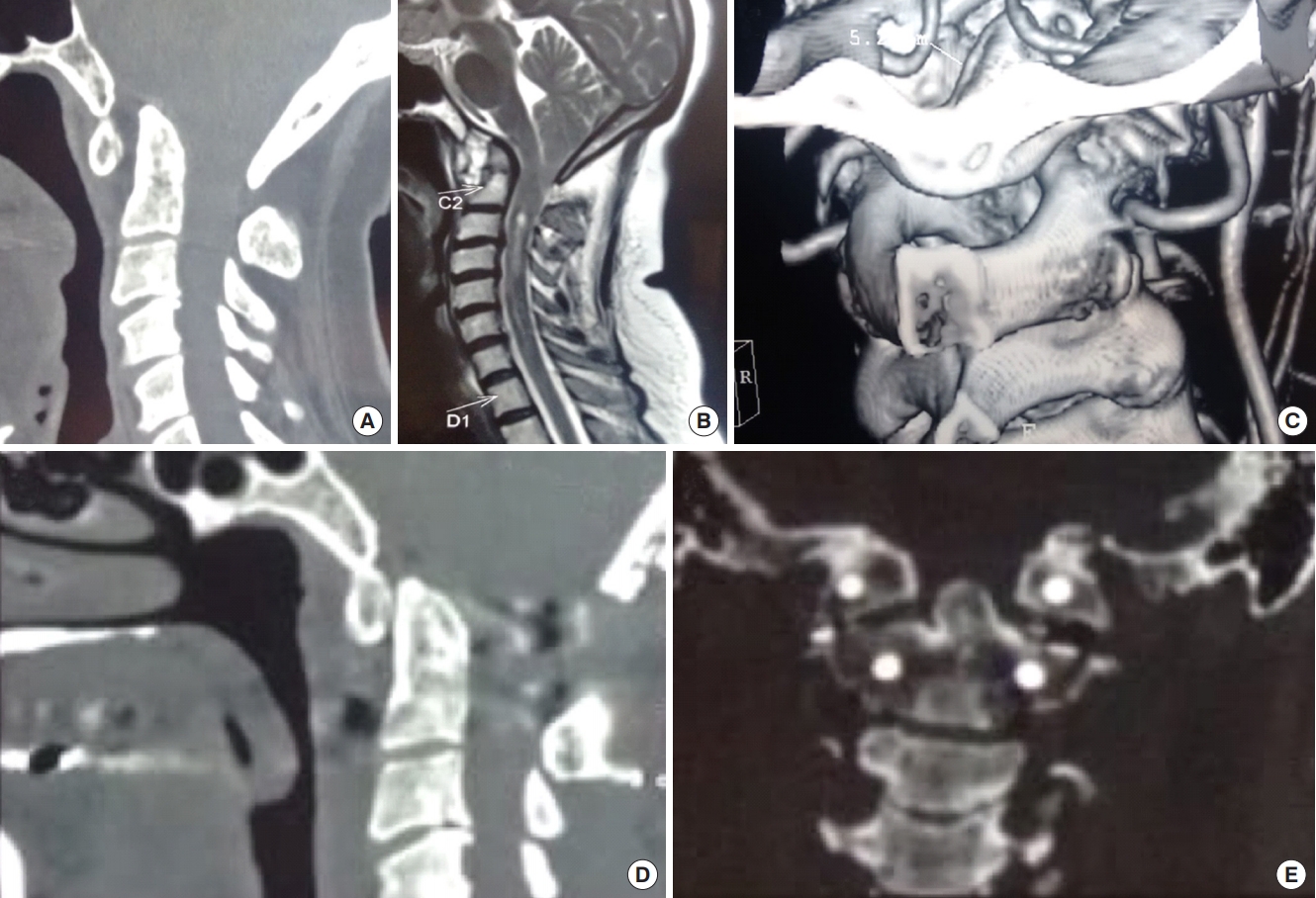
Fig. 3.
(A) T2-weighted sagittal magnetic resonance image of the spine showing Chiari I malformation with syringomyelia in which posterior fossa decompression has been performed. (B) Midsagittal computed tomography (CT) reconstruction showing no atlantoaxial dislocation and the removal of posterior arch of C1 and posterior rim of the foramen magnum. (C) Coronal CT scan showing nearly symmetrical facet joints. In this patient, posterior fossa decompression resulted in resolution of symptoms and a decrease of syringomyelia.
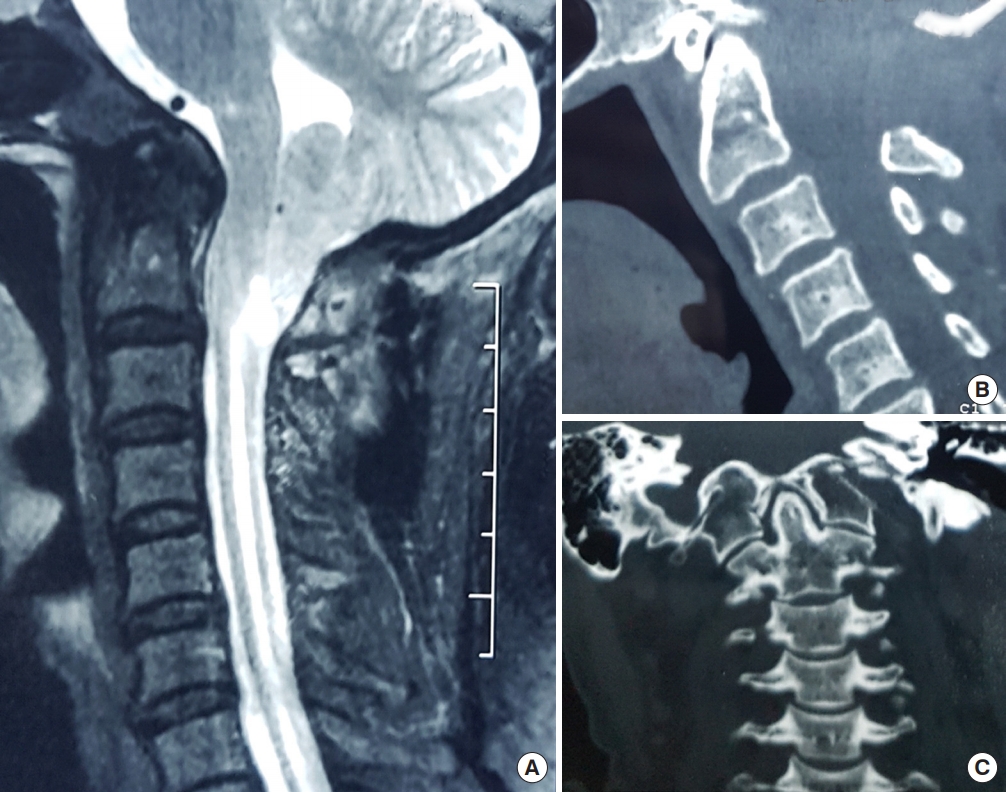
Fig. 4.
(A) T2-weighted sagittal magnetic resonance image of the spine showing Chiari I malformation with syringomyelia. (B) Midsagittal computed tomography (CT) reconstruction in flexion. (C) Midsagittal CT reconstruction in extension showing no atlantoaxial dislocation. (D) Coronal CT scan showing symmetrical facet joints. As this was a patient with pure Chiari I malformation with no bone instability and symmetrical facet joints, only posterior fossa decompression/augmentation with or without duroplasty was mandated.
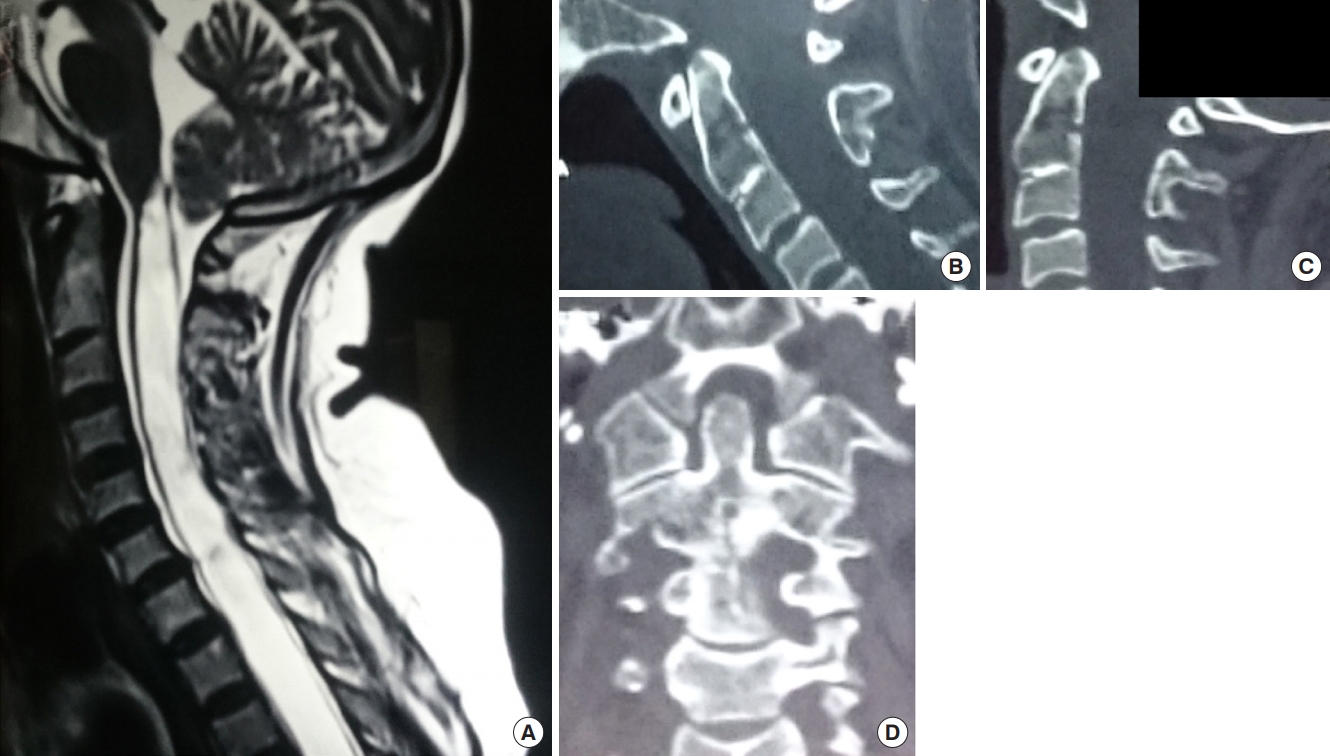
Fig. 5.
Variations in the third part of the vertebral artery that brings it just posterior to the C1–2 facet joint and makes it vulnerable to injury. (A) Normal course of the vertebral artery. (B) Entry site of the vertebral artery from a congenital foramen in the occipitalized atlas. (C) Low lying posterior inferior cerebellar artery. (D) Persistent first intersegmental artery. (E) Fenestrated vertebral artery. Reprinted from Sardhara et al. Neurol India 2015;63:382-91, with permission of Neurology India [38].
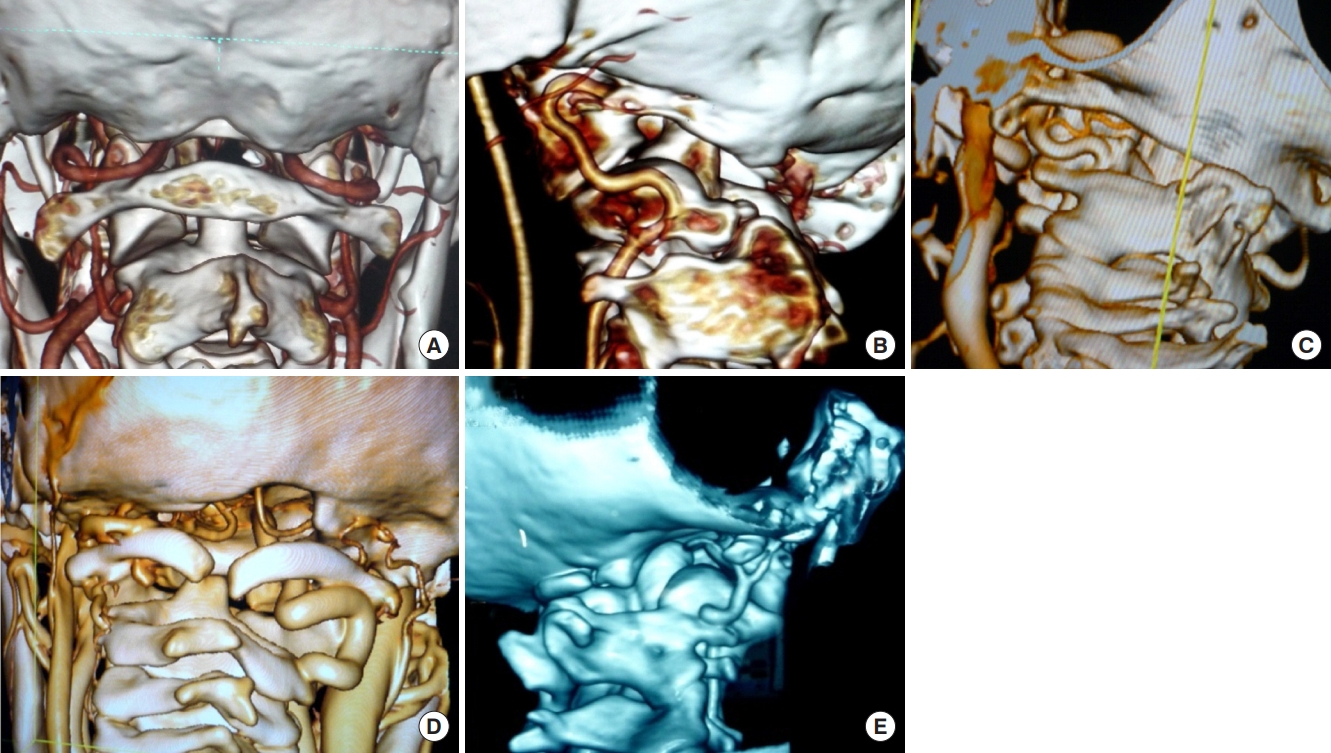
Fig. 6.
(A) Sagittal reconstructed computed tomography (CT) scan image showing atlantoaxial dislocation with C2–3 fusion with canal compromise at foramen magnum. (B) Coronal CT scan image showing grossly asymmetrical facet joints. (C) Three-dimensional coronal CT scan images showing asymmetrical C1–2 facet joints with hypoplasia of unilateral vertebral artery. (D) Postoperative image after C1–2 facet joint distraction and lateral mass rod and screw fixation (E, F) showing an overdistracted axis vertebra well below the C1 anterior arch. Due to this overdistraaction, the patient had neurological deterioration that only partially recovered with loosening of the construct and its retightening following permitting of axis and atlas to retain their ‘in situ’ position.
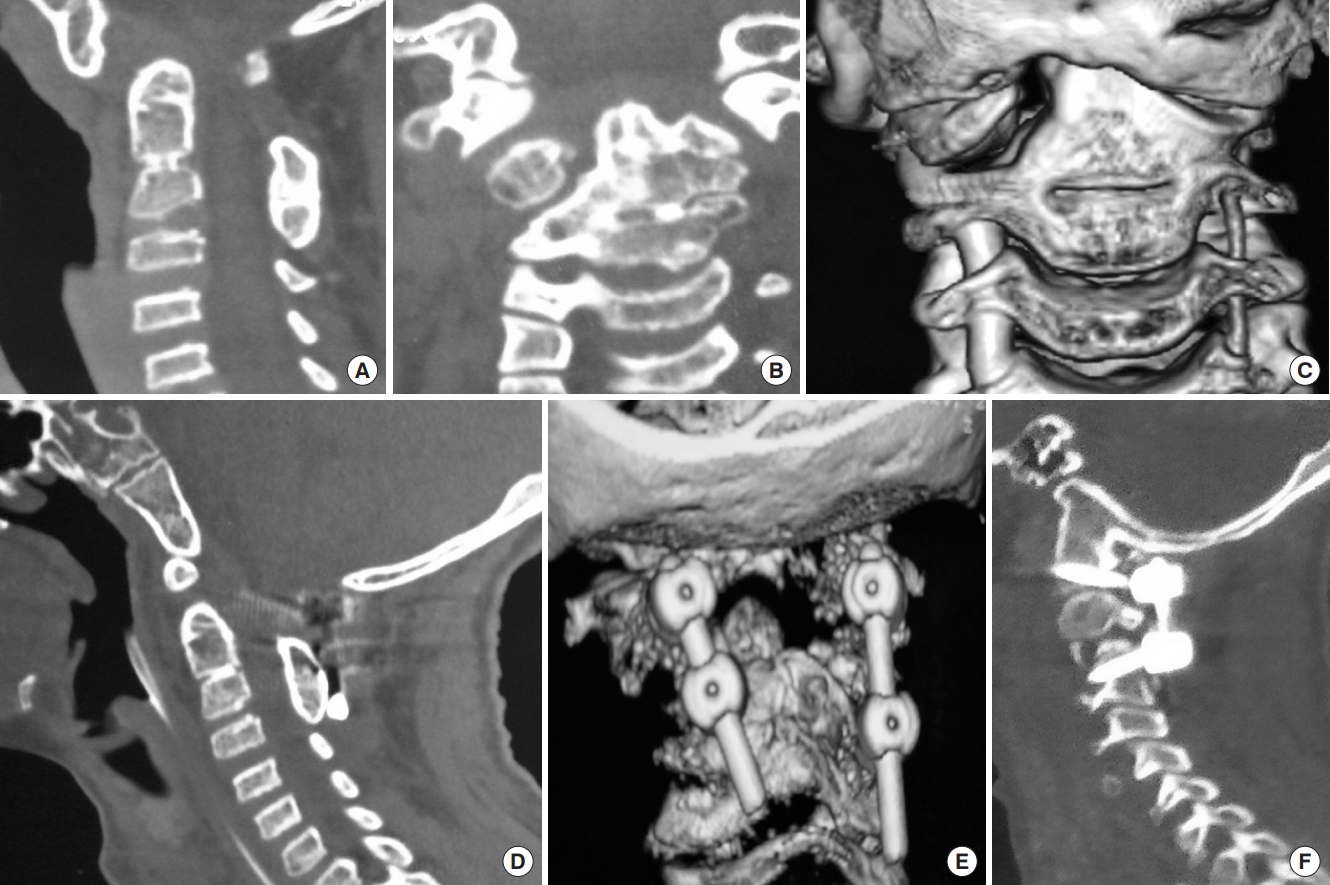
Fig. 7.
(A) Sagittal reconstructed computed tomography (CT) scan image showing atlantoaxial dislocation (AAD) with C2–3 fusion with bifid posterior C1 arch causing canal compromise at foramen magnum. (B) Coronal CT scan image showing grossly asymmetrical facet joints with scoliosis and lateral cervical tilt. (C) Three-dimensional coronal CT scan images showing asymmetrical C1–2 facet joints with bilateral nearly equal caliber of the vertebral arteries. (D, E) Following C1–2 distraction and posterior stabilization, there was still persistent AAD causing canal compromise due to which the patient had neurological deterioration. A part of the neurological deterioration may have been due to spinal cord torsion due to the attempt to correct the bony deformity to its physiological position. (F) Following a transoral decompression in the second stage, the patient regained significant power and was discharged with persistent myelopathy
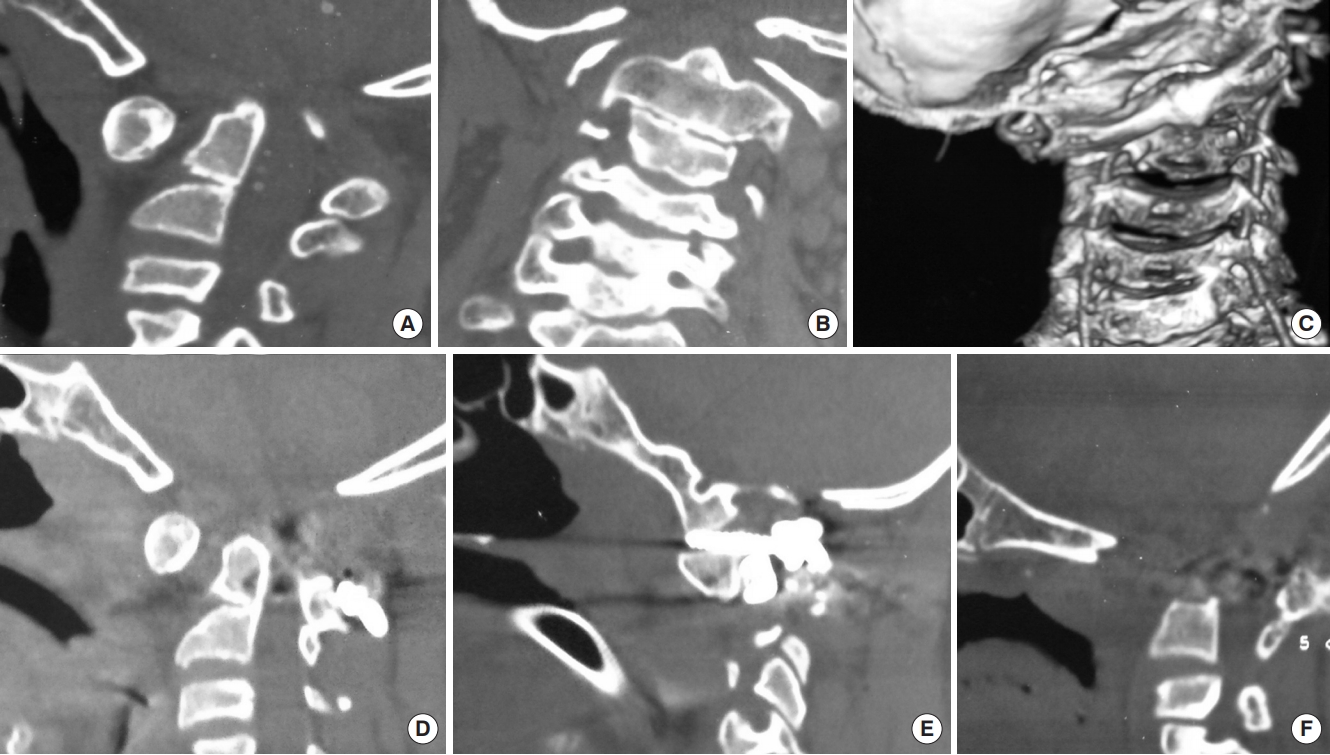
Table 1.
Review of the large surgical series of posterior fossa decompression and its outcome in adult cases of Chiari I malformation
| Study | No. of cases | Surgery | Improve- ment (%) | Stable (%) | Deteriora- tion (%) | Follow-up (mo) | Deaths (%) |
|---|---|---|---|---|---|---|---|
| Gardner, 1977 [3] | 74 | PFD | 69.5 | NA | 30.5 | NA | 6.7 |
| Hurth, 1979 [11] | 63 | PFD | 54.6 | 31.8 | 13.6 | NA | 1.6 |
| Levy et al., 1983 [12] | 85 | PFD | 40 | 27 | 26 | 42 | 4.7 |
| Matsumoto and Symon, 1989 [13] | 38 | PFD | 58.3 | NA | 38.4 | 70 | 3.3 |
| Pillay et al., 1991 [14] | 20 | PFD | 45 | 46 | 10 | 40 | NA |
| Morgan and Williams, 1992 [15] | 54 | PFD | 83 | 9 | 8 | 168 | NA |
| Versari et al., 1993 [16] | 40 | PFD | 63 | 23 | 15 | 84 | 0 |
| Williams, 1993 [17] | 54 | PFD | 83 | 9 | 8 | NA | 0 |
| Tognetti and Calbucci, 1983 [18] | 17 | PFD | 82 | 12 | 6 | 18 | 0 |
| Hida et al., 1995 [19] | 33 | PFD | 82 | NA | NA | 38 | 0.75 |
| Klekamp et al., 1996 [20] | 44 | PFD | 86 | NA | NA | 39 | 5.4 |
| Guyotat et al., 1998 [21] | 66 | PFD | 47 | 24 | 22 | 52 | 2 |
| Blagodatsky et al., 1999 [22] | 102 | PFD | 67.6 | 20 | 9.8 | NA | 0.7 |
| Aghakhani et al., 1999 [23] | 242 | PFD | 38 | 50 | 12 | 72 | NA |
| Munshi et al., 2000 [24] | 11/23 | PFD/PFD with duroplasty | 72.7/86.9 | NA | 27.3/13.1 | 6 | 0 |
| Arora et al., 2004 [25] | 58 | PFD | 62.5 | 37.5 | NA | 6 | 0.8 |
| Zhang et al., 2008 [26] | 236 | PFD | 80.69 | 15.51 | 3.8 | NA | 1.3 |
| Teo et al., 2018 [27] | 14 | PFD | 71.4 | 14.3 | 14.3 | NA | NA |
Table 2.
Case-based recommendation for patients with Chiari I malformation
REFERENCES
1. Milhorat TH, Chou MW, Trinidad EM, et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery 1999 44:1005-17.


2. Chiari H. Uber Veränderungen des Kleinhirns, des Pons und der Medulla Oblongata infolge von kongenitaler Hydrocephalie des Grosshirns. Denkschr Akad Wiss Wien 1891 63:71-116.
3. Gardner WJ. Syringomyelia. Surg Neurol 1977 7:370.
4. Gardner WJ, McMurray FG. “Non-communicating” syringomyelia: a non-existent entity. Surg Neurol 1976 6:251-6.

5. Williams B. A critical appraisal of posterior fossa surgery for communicating syringomyelia. Brain 1978 101:223-50.



7. Aboulker J. Syringomyelia and intra-rachidian fluids. X. Rachidian fluid stasis. Neurochirurgie 1979 25 Suppl 1:98-107.

8. Oldfield EH, Muraszko K, Shawker TH, et al. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J Neurosurg 1994 80:3-15.


9. Oldfield EH. Pathogenesis of Chiari I - Pathophysiology of syringomyelia: implications for therapy: a summary of 3 decades of clinical research. Neurosurgery 2017 64(CN_suppl):66-77.



10. Goel A. Cervical fusion as a protective response to craniovertebral junction instability: a novel concept. Neurospine 2018 15:323-8.




11. Hurth M. Surgery of craniocervical junction: Analysis of a surgical series of 63 operated syringomyelia. Neurochirurgie 1979 25:114. -28. French.
12. Levy WJ, Mason L, Hahn JF. Chiari malformation presenting in adults: a surgical experience in 127 cases. Neurosurgery 1983 12:377-90.


13. Matsumoto T, Symon L. Surgical management of syringomyelia--current results. Surg Neurol 1989 32:258-65.


14. Pillay PK, Awad IA, Little JR, et al. Symptomatic Chiari malformation in adults: a new classification based on magnetic resonance imaging with clinical and prognostic significance. Neurosurgery 1991 28:639-45.


15. Morgan D, Williams B. Syringobulbia: a surgical appraisal. J Neurol Neurosurg Psychiatry 1992 55:1132-41.



16. Versari PP, D’Aliberti G, Talamonti G, et al. Foraminal syringomyelia: suggestion for a grading system. Acta Neurochir (Wien) 1993 125:97-104.



17. Williams B. Surgery for hindbrain related syringomyelia. Adv Tech Stand Neurosurg 1993 20:107-64.


18. Tognetti F, Calbucci F. Syringomyelia: syringo-subarachnoid shunt versus posterior fossa decompression. Acta Neurochir (Wien) 1993 123:196-7.

19. Hida K, Iwasaki Y, Koyanagi I, et al. Surgical indication and results of foramen magnum decompression versus syringosubarachnoid shunting for syringomyelia associated with Chiari I malformation. Neurosurgery 1995 37:673-8.



20. Klekamp J, Batzdorf U, Samii M, et al. The surgical treatment of Chiari I malformation. Acta Neurochir (Wien) 1996 138:788-801.



21. Guyotat J, Bret P, Jouanneau E, et al. Syringomyelia associated with type I Chiari malformation. A 21-year retrospective study on 75 cases treated by foramen magnum decompression with a special emphasis on the value of tonsils resection. Acta Neurochir (Wien) 1998 140:745-54.



22. Blagodatsky MD, Larionov SN, Alexandrov YA, et al. Surgical treatment of Chiari I malformation with or without syringomyelia. Acta Neurochir (Wien) 1999 141:963-8.



23. Aghakhani N, Parker F, Tadié M. Syringomyelia and Chiari abnormality in the adult. Analysis of the results of a cooperative series of 285 cases. Neurochirurgie 1999 45 Suppl 1:23-36.

24. Munshi I, Frim D, Stine-Reyes R, et al. Effects of posterior fossa decompression with and without duraplasty on Chiari malformation-associated hydromyelia. Neurosurgery 2000 46:1384-9.



25. Arora P, Behari S, Banerji D, et al. Factors influencing the outcome in symptomatic Chiari I malformation. Neurol India 2004 52:470-4.

26. Zhang ZQ, Chen YQ, Chen YA, et al. Chiari I malformation associated with syringomyelia: a retrospective study of 316 surgically treated patients. Spinal Cord 2008 46:358-63.



27. Teo KA, Yang L, Leow R, et al. Minimally-invasive approach to posterior fossa decompression: Initial experience in adult Chiari type 1 malformation patients. J Clin Neurosci 2018 56:90-4.


28. Chai Z, Xue X, Fan H, et al. Efficacy of posterior fossa decompression with duraplasty for patients with Chiari malformation type I: a systematic review and meta-analysis. World Neurosurg 2018 113:357. -65. e1.


29. Arora P, Pradhan PK, Behari S, et al. Chiari I malformation related syringomyelia: radionuclide cisternography as a predictor of outcome. Acta Neurochir (Wien) 2004 146:119-30.



30. Behari S, Kalra SK, Kiran Kumar MV, et al. Chiari I malformation associated with atlanto-axial dislocation: focussing on the anterior cervico-medullary compression. Acta Neurochir (Wien) 2007 149:41-50.



31. Klekamp J, Samii M. Introduction of a score system for the clinical evaluation of patients with spinal processes. Acta Neurochir (Wien) 1993 123:221-3.

32. Behari S, Bhargava V, Nayak S, et al. Congenital reducible atlantoaxial dislocation: classification and surgical considerations. Acta Neurochir (Wien) 2002 144:1165-77.



33. Potgieser AR, Hoving EW. A novel technique to treat acquired Chiari I malformation after supratentorial shunting. Childs Nerv Syst 2016 32:1721-5.




34. Anderson PA, Oza AL, Puschak TJ, et al. Biomechanics of occipitocervical fixation. Spine (Phila Pa 1976) 2006 31:755-61.


35. Kalra SK, Jain VK, Jaiswal AK, et al. Occipitocervical contoured rod stabilization: does it still have a role amidst the modern stabilization techniques? Neurol India 2007 55:363-8.


36. Bick S, Dunn R. Occipito-cervical fusion: review of surgical indications, techniques and clinical outcomes. SA Orthop J 2010 9:26-32.
37. Sardhara J, Behari S, Sindgikar P, et al. Evaluating atlantoaxial dislocation based on Cartesian coordinates: proposing a new definition and its impact on assessment of congenital torticollis. Neurosurgery 2018 82:525-40.



38. Sardhara J, Behari S, Mohan BM, et al. Risk stratification of vertebral artery vulnerability during surgery for congenital atlanto-axial dislocation with or without an occipitalized atlas. Neurol India 2015 63:382-91.


39. Sindgikar P, Das KK, Sardhara J, et al. Craniovertebral junction anomalies: when is resurgery required? Neurol India 2016 64:1220-32.


40. Das KK, Sindgikar P, Srivastava A, et al. Elongated clivus with deficient anterior atlantal arch and congenital posterior atlanto-occipital dislocation: patho-embryology and management nuances of a rare form of proatlas segmentation anomaly. World Neurosurg 2019 Mar 18 pii: S1878-8750(19)30759-4.


- TOOLS
- Related articles in NS
-
Journal Impact Factor 3.2




























