 |
 |
- Search
|
|
||
Abstract
Objective
Methods
Results
NOTES
Conflict of Interest
Jin-Sung Kim is a consultant of RIWOSpine, GmbH, Germany; Elliquence, LLC, USA; and Stöckli Medical AG, Switzerland. The other authors have no conflicts of interest to declare.
Fig. 2.

Fig. 3.
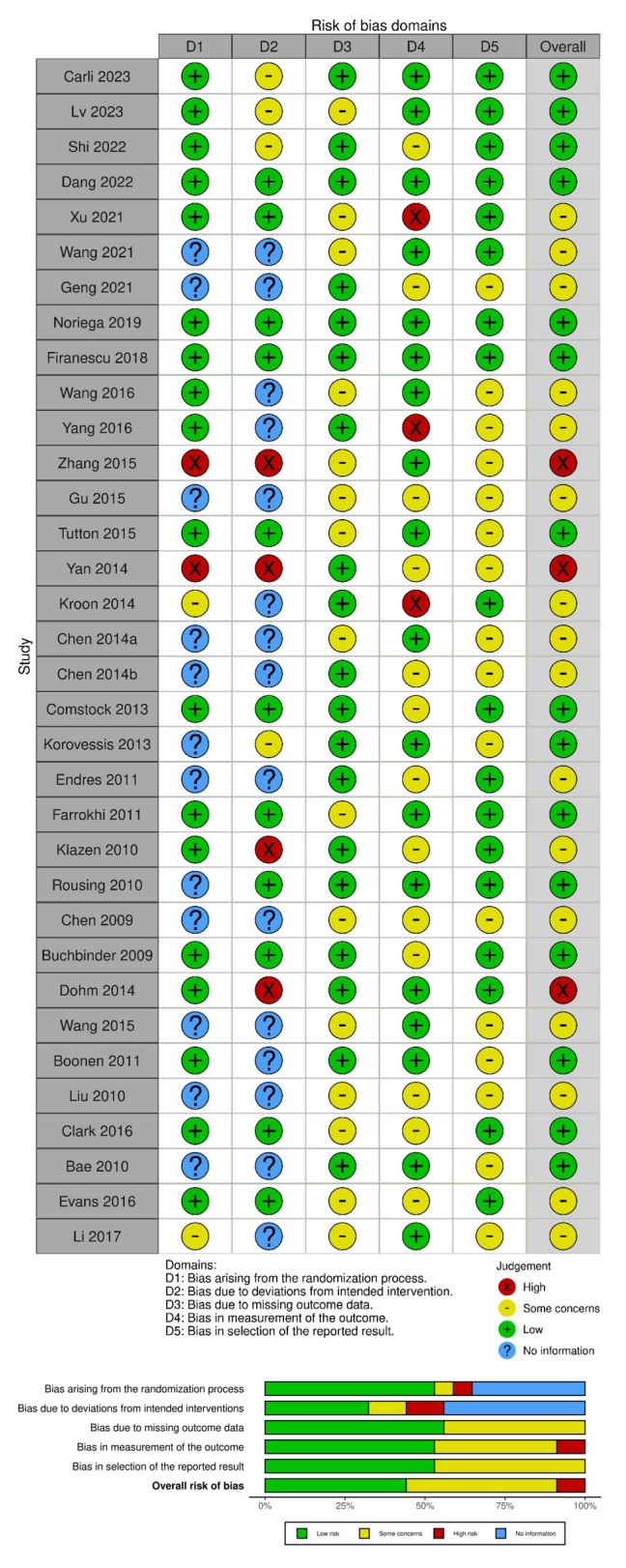
Fig. 4.

Fig. 5.
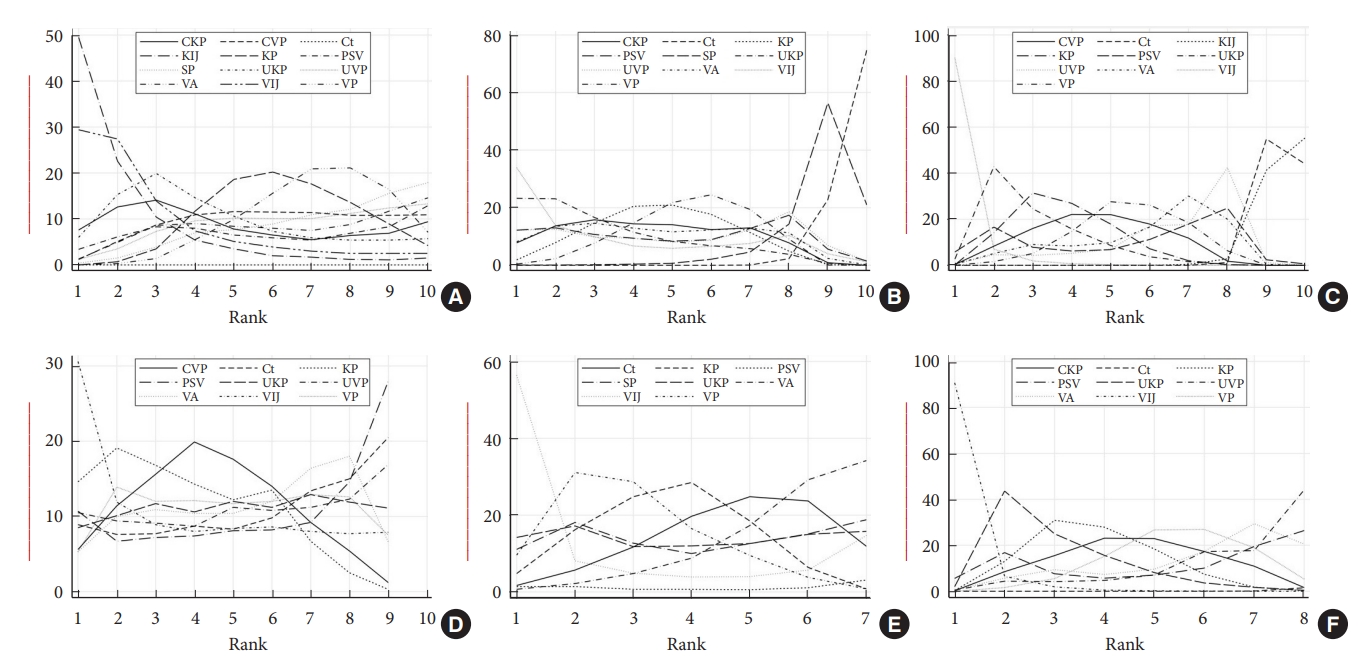
Fig. 6.

Fig. 7.
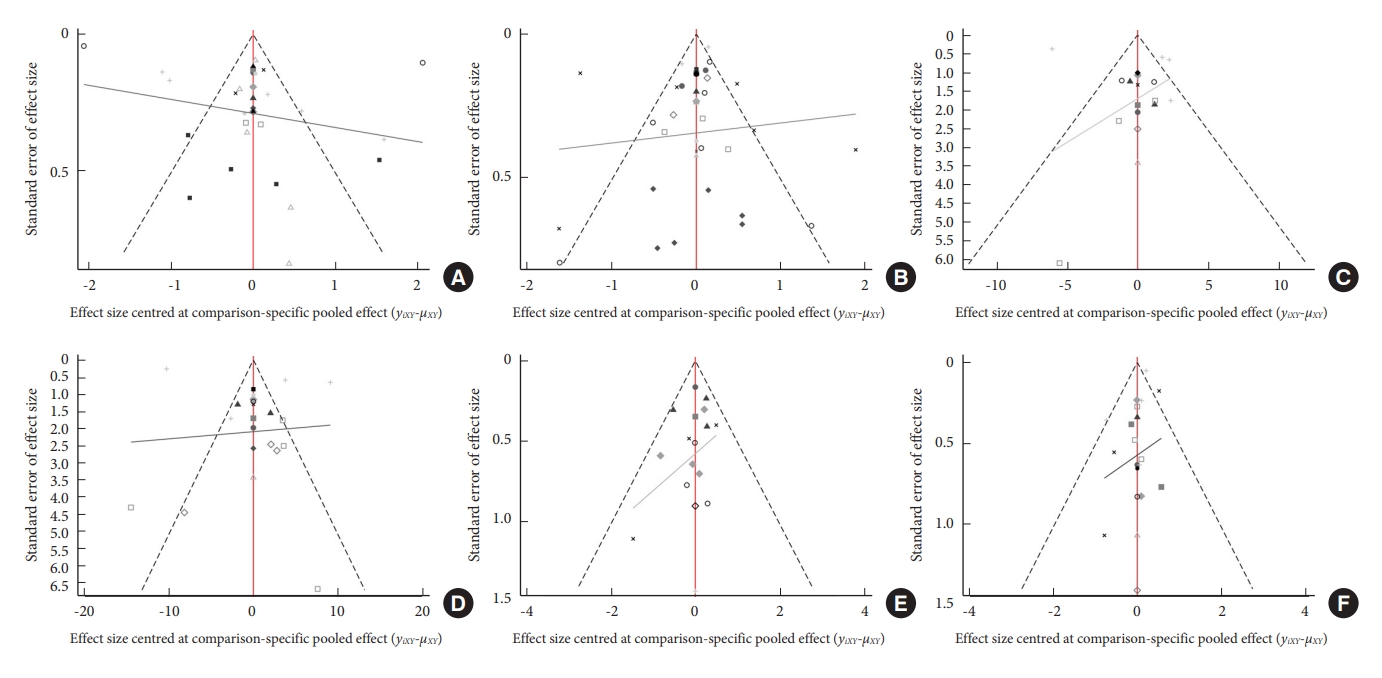
Table 1.
| No. | Study | Identifier | Journal | Country | Year | Time | Center | Interventions | Control | Follow-up | N/Female | Age (yr) | Outcomes measurements |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Carli et al. [14] | NCT 01963039 | Radiology | Netherlands | 2023 | May 2013 to June 2019 | Single-center | Vertebroplasty | Sham placebo | 1 day, 1 wk, 1, 3, 6, 12 mo | 80/54 | 69 ± 10 | VAS, QUALEFFO, RMDQ, analgesics use, fracture cleft, new fractures |
| 2 | Lv et al. [15] | ChiCTR 2000036792 | Spine | China | 2023 | September 2020 to April 2022 | Single-center | Curved vertebroplasty | Unipedicular vertebroplasty | 1 day, 1 wk, 1, 3, 6, 12 mo | 103/59 | 74.81 ± 5.87 | VAS, ODI, cement leakage, cement distribution, cement volume, fluoroscopy times, operation duration |
| 3 | Shi et al. [16] | ChiCTR 2200056785 | The spine journal | China | 2022 | May 2019 to October 2020 | Single-center | Curved kyphoplasty | Bilateral kyphoplasty | 1 day, 6, 12 mo | 85/71 | 70.99 ± 6.79 | VAS, ODI, anterior edge height, kyphosis angulation, cement distribution, cement leakage, leakage pattern, frequency of intraoperative fluoroscopy, operative time, cement volume |
| 4 | Dang et al. [17] | ChiCTR 2200056526 | BMC Musculoskeletal Disorders | China | 2022 | January 2018 to December 2020 | Single-center | Vertebroplasty with facet joint block | Vertebroplasty | 1 day, 1, 3, 6, 12 mo | 198/103 | 77.39 ± 7.78 | VAS, ODI, cement volume, hospitalization time, operation time, cement volume complication rate, Cox-2 inhibitors |
| 5 | Xu et al. [18] | SAHoWMU-CR2018-08-109 | Pain Physician | China | 2021 | August 2019 to November 2019 | Single-center | Kyphoplasty with medial branch block | Kyphoplasty | 1 day, 1 wk, 1, 3, 6, 12 mo | 179/151 | N/A | VAS, PROMIS PF, satisfaction, the incidence of residual back pain |
| 6 | Wang et al. [19] | ChiCTR 2100042859 | BMC Musculoskeletal Disorders | China | 2021 | January 2019 to January 2020 | Single-center | Curved vertebroplasty | Kyphoplasty | 1 day, 6 mo | 72/50 | 76.04 ± 6.15 | VAS, ODI, fluoroscopy times, cement volume, anterior edge height, Cobb angle, cement leakage |
| 7 | Geng et al. [20] | N/A | Orthopedics | China | 2021 | February 2016 to February 2018 | Single-center | Curved vertebroplasty | Vertebroplasty | 2 day, 3 mo | 96/64 | 70.56 ± 5.91 | VAS, ODI, Cobb angle, vertebral body height variation |
| 8 | Noriega et al. [21] | NCT02461810 | The spine journal | Spain | 2019 | April 2015 to February 2017 | Multi-center | Titanium vertebral augmentation | kyphoplasty | 5 day, 1, 6, 12 mo | 141/111 | 73.3 ± 9.5 | Pain intensity, BMD, ODI, EQ-5D, ambulatory status, analgesic consumption, length of hospital stay, symptomatic cement extravasation requiring surgical reintervention or retreatment |
| 9 | Firanescu et al. [22] | NCT01200277 | the BMJ | Netherlands | 2018 | January 2011 to January 2013 | Multi-center | Vertebroplasty | Sham placebo | 1 day, 1 wk, 1, 3, 6, 12 mo | 176/133 | 75.78 ± 9.56 | VAS, QUALEFFO, RMDQ |
| 10 | Li et al. [23] | N/A | Int J Clin Exp Med | China | 2017 | January 2013 to June 2015 | Single-center | Kyphoplasty | Conservative | 1 wk, 1, 3, 6 mo | 80/24 | 74.03 ± 6.21 | VAS, ODI, Cobb angle, vertebral height, vertebral fracture restoration, symptom relief |
| 11 | Wang et al. [24] | N/A | Eur Spine J | China | 2016 | January 2009 and January 2013 | Single-center | Facet joint block | Vertebroplasty | 1 day, 1 wk, 1, 3, 6, 12 mo | 200/165 | 63.14 ± 5.56 | VAS, ODI, BMD, SF-36, new fractures |
| 12 | Yang et al. [25] | N/A | Spine | China | 2016 | January 2009 to December 2011 | Single-center | Vertebroplasty | Conservative treatment | 1 day, 1 wk, 1, 3, 6, 12 mo | 107/69 | 76.67 ± 5.80 | VAS, ODI, QUALEFFO, satisfaction surveys |
| 13 | Clark et al. [26] | NCT01482793 | Lancet | Australia | 2016 | Nov 2011 to Dec 2014 | Multi-center | Vertebroplasty | Sham placebo | 3, 14 day, 1, 3, 6 mo | 120/88 | 80.49 ± 6.99 | NRS, RMDQ, QUALEFFO, EQ-5D, analgesic use |
| 14 | Evans et al. [27] | NCT00279877 | Journal of NeuroInterventional Surgery | United States | 2016 | N/A | Multi-center | Vertebroplasty | Kyphoplasty | 3, 30 day, 6, 12 mo | 115/82 | 75.6 ± 10.0 | VAS, RMDQ, SOF-6 ADL, EQ-5D, SF-36, OPAQ |
| 15 | Zhang et al. [28] | N/A | BMC Musculoskeletal Disorders | China | 2015 | November 2010 to October 2012 | Single-center | Uni-vertebroplasty | Bi-vertebroplasty | 3 day, 24 mo | 50/ 37 | 71.91 ± 6.69 | VAS, ODI, SF-36, anterior/middle vertebral body height variation, Cement leakage, Cement volume, operation time, radiation time, incidence of complications |
| 16 | Gu et al. [29] | N/A | Journal of Orthopaedic Surgery and Research | China | 2015 | November 2010 to August 2011 | Single-center | Pedicle screw fixation | Vertebroplasty | 1, 2, 3, 6, 12, 24 mo | 68/47 | 74.55 ± 5.91 | VAS, anterior/central vertebral body height, local kyphosis, Cobb angle |
| 17 | Tutton et al. [30] | N/A | Spine | United States | 2015 | August 2010 to May 2013 | Multi-center | Kiva Augmentation Treatment | Kyphoplasty | 30 day, 6, 12 mo | 285/211 | 75.56 ± 9.22 | VAS, ODI, DXA, cement volume, cement extravasation, absence of device-related SAEs, adjacent fracture |
| 18 | Wang et al. [31] | N/A | Pain Physician | China | 2015 | January 2012 to February 2014 | Single-center | Vertebroplasty | Kyphoplasty | 1 day, 3, 12 mo | 107/81 | 69.03 ± 8.64 | VAS, ODI, location of leakages, cement leakage, and vertebral height restoration, cement volumes |
| 19 | Yan et al. [32] | N/A | Spine | China | 2014 | January 2009 to January 2012 | Single-center | Uni-kyphoplasty | Bi-kyphoplasty | 1, 3, 6, 12 mo | 309/220 | 71.51 ± 3.98 | VAS, SF-36, RMDQ, cement volume, radiation dose, anterior/posterior vertebral body height, kyphotic angle, cement distribution |
| 20 | Kroon et al. [33] | ACTRN01260 5000079640 | Journal of Bone and Mineral Research | Australia | 2014 | April 2004 to October 2008 | Multi-center | Vertebroplasty | Sham | 1 wk, 1, 3, 6, 12, 24 mo | 57/47 | 77.19 ± 9.23 | Pain intensity, QUALEFFP, AQoL, RMDQ, EQ-5D, timed up & go, cement volume, pain at rest and at night, disability, quality of life, perceived recovery, adverse events |
| 21 | Chen et al. [34] | N/A | Journal of spinal disorders & techniques | China | 2014 | N/A | Single-center | Uni-vertebroplasty | Bi-vertebroplasty | 2 wk, 24 mo | 39/N/A | 69.06 ± 7.49 | VAS, ODI, cement volume, cement leakage |
| 22 | Chen et al. [35] | N/A | Journal of Clinical Neuroscience | China | 2014 | January 2007 to December 2012 | Single-center | Vertebroplasty | Conservative | 1 wk, 1, 3, 6, 12 mo | 89/62 | 65.53 ± 9.10 | VAS, ODI, RMDQ, drugs control, pain relief, functional outcome |
| 23 | Dohm et al. [36] | NCT00323609 | Am J Neuroradiol | United States | 2014 | October 2006 to May 2011 | Multi-center | Vertebroplasty | Kyphoplasty | 1 day, 3, 12, 24 mo | 361/280 | 75.6 | VAS, ODI, SF-36, EQ-5D, cement volume, cement leakage, radiographic fracture, new radiographic VCF, kyphotic angulation |
| 24 | Comstock et al. [37] | NCT00068822 | Neuroradiology | United States | 2013 | June 2004 to August 2008 | Multi-center | Vertebroplasty | Sham placebo | 1, 3, 6, 12 mo | 131/N/A | N/A | Pain intensity, RMDQ |
| 25 | Korovessis et al. [38] | N/A | J Bone & Mineral Research (Spine) | Greece | 2013 | May 2010 to September 2010 | Single-center | Kiva Aug-mentation Treatment | Kyphoplasty | 12 mo | 168/119 | 70.98 ± 10.34 | VAS, SF-36, ODI, anterior/midline/posterior vertebra body height ratio, cement leakage, Gardner angle |
| 26 | Endres et al. [39] | AZ 2010-218-f-s | Orthopaedics & Traumatology: Surgery & Research | Germany | 2011 | N/A | Single-center | Soteira shield kyphoplasty system | Kyphoplasty/Vertebroplasty | 6 mo | 59/40 | 53–77 | VAS, ODI, dose-area product, beck index, fluoroscopy times, radiological imaging with regard to height restoration, cement distribution, cement leakage, recurrent fracture |
| 27 | Farrokhi et al. [40] | IRCT13880 4252193N1 | J Neurosurg Spine | Iran | 2011 | September 2004 to January 2006 | Single-center | Vertebroplasty | Conservative | 1 wk, 2, 6, 12, 24, 36 mo | 82/60 | 55–90 | VAS, ODI, vertebra body height, kyphotic angulation, sagittal index |
| 28 | Boonen et al. [41] | N/A | Journal of Bone and Mineral Research | United Kingdom | 2011 | February 2003 to December 2005 | Multi-center | Kyphoplasty | Conservative | 7 day, 1, 3, 6, 12, 24 mo | 300/232 | 44.5–95.2 | VAS, SF-36, RMDQ, reduced activity, EQ-5D, limited days of activity and bed rest, patient satisfaction (20-point Likert scale) |
| 29 | Klazen et al. [42] | NCT00232466 | Lancet | Netherlands | 2010 | October 2005 to June 2008 | Multi-center | Vertebroplasty | Conservative | 1 day, 1 wk, 1, 3, 6, 12 mo | 202/140 | 75.30 ± 9.10 | VAS, QUALEFFO, EQ-5D, RMDQ, medical costs, pain relief, cost-effectiveness, QALYs, RMDQ |
| 30 | Rousing et al. [43] | 20000161-20030072 | Spine | Denmark | 2010 | January 2001 to January 2008 | Single-center | Vertebroplasty | Conservative | 3, 12 mo | 49/40 | 65–96 | VAS, SF-36, DPQ, EQ-5D, Barthel, MMSE, tandem test, timed up & go, chair test |
| 31 | Liu et al. [44] | N/A | Osteoporos Int | China | 2010 | N/A | Single-center | Vertebroplasty | Kyphoplasty | 3 day, 6 mo | 100/77 | 57–84 | VAS, vertebral body height, kyphotic wedge angle, location of osteoporotic VCFs, period between injury and surgery |
| 32 | Bae et al. [45] | N/A | Spine | United Kingdom | 2010 | N/A | Multi-center | Vertebroplasty | Kyphoplasty | 1 wk, 1, 3, 6, 12, 24, 36 mo | 40/26 | 75.5 ± 12.15 | VAS, ODI, SF-12, cement volume |
| 33 | Chen et al. [46] | N/A | Injury | China | 2009 | N/A | Single-center | Uni-kyphoplasty | Bi-kyphoplasty | 2 wk | 58/N/A | 68.07 ± 7.09 | VAS, ODI, restoration rate, surgery time, vertebral height changes, clinical outcomes |
| 34 | Buchbinder et al. [47] | ACTRN0126050 00079640 | N Engl J Med | Australia | 2009 | April 2004 to October 2008 | Multi-center | Vertebroplasty | Sham placebo | 1 wk, 1, 3, 6 mo | 78/62 | 76.61 ± 12.06 | Pain intensity, QUALEFFP, AQoL, RMDQ, EQ-5D |
VAS, visual analogue scale; QUALEFFO, quality of life questionnaire; RMDQ, Roland Morris Disability Questionnaire; ODI, Oswestry Disability Index; NRS, Numerical Rating Scale; PROMIS PF, patient-reported outcomes measurement information system physical function; BMD, bone mineral density; EQ-5D, European quality of life–5 dimensions; SF-36, 36-item Short Form health survey; SOF ADL, Study of Osteoporotic Fractures Index Scoring activities of daily living; OPAQ, Osteoporosis Assessment Questionnaire; DXA, dual-energy x-ray absorptiometry; AQoL, assessment of quality of life; VCF, vertebral compression fracture; QALYs, quality-adjusted life years; SF-12, 12-item Short Form health survey; DPQ, Dallas Pain Questionnaire; MMSE, modified mini-mental state examination.
REFERENCES

- TOOLS
-
METRICS

-
- 1 Crossref
- Scopus
- 1,071 View
- 133 Download
- Related articles in NS
-
Journal Impact Factor 3.2



























