 |
 |
- Search
|
|
||
Abstract
Objective
There is a lack of literature on indirect decompression in uniportal endoscopic posterolateral transforaminal lumbar interbody fusion (EPTLIF). Our aim is to evaluate the dimensions of the spinal canal and contralateral foramen before and after EPTLIF.
Methods
This is a retrospective study of patients who underwent EPTLIF in a tertiary spine centre over a 2-year period. The cross-sectional area of the spinal canal and the contralateral foramen at the level of fusion were measured on magnetic resonance imaging scan at 1-day postoperation and at the final follow-up. Patients were grouped according to the decompression performed as per the clinician’s judgement.
Results
One hundred fifty-two levels of fusion were performed in 120 patients. There was a statistically significant clinical improvement in visual analogue scale and Oswestry Disability Index scores postoperation. The measurements of the spinal canal area were 106.0 mm2, 138.8 mm2, and 195.5 mm2; while contralateral foraminal area were 73.2 mm2, 104.4 mm2, and 120.7 mm2 at preoperation, 1-day postoperation, and at the final follow-up, respectively (p < 0.001). For the subgroup analyses, spinal canal area measurements for the bilateral decompression cohort (n = 35) were 57.0 mm2, 123.9 mm2, and 191.8 mm2; for the ipsilateral decompression cohort (n = 42) were 89.3 mm2, 128.9 mm2, 183.3 mm2; and for the cohort without any decompression and only cage inserted (n = 75) were 138.3 mm2, 151.2 mm2, and 204.1 mm2 (p < 0.001). Contralateral foraminal area measurements were 73.3 mm2, 106.4 mm2 and 120.4 mm2 in the bilateral decompression cohort; 69.5 mm2, 99.0 mm2, 116.9 mm2 in the ipsilateral decompression cohort; and 75.1 mm2, 106.5 mm2, 122.9 mm2 in the cohort without any decompression (p < 0.001).
Lumbar spinal fusion surgeries have been the mainstay of treatment for many degenerative lumbar spinal conditions such as lumbar spinal stenosis and spondylolisthesis with dynamic instability [1-3]. With better understanding of the anatomy and invention of more advanced endoscopic instruments and techniques, there has been a push for minimally invasive techniques to help preserve the normal anatomy and musculature of the lumbar spine in order to prevent the increased morbidity and reduce the risks of adjacent segment disease [4,5] seen in traditional open methods such as the transforaminal lumbar interbody fusion (TLIF) technique described by Harms [6].
In cases with foraminal stenosis, direct decompression requires additional bone and soft tissue (on top of those required for fusion alone) would need to be removed. This may increase the instability of the adjacent level, resulting in a theoretical increased risk of adjacent segment disease [7,8]. Over aggressive decompression may also result in iatrogenic injuries to the neural elements, dural tears, and epidural haematoma. This is especially seen in nonendoscopic minimally invasive surgical (MIS) techniques which has been shown to have a higher rate of nerve root injuries when compared to traditional open techniques [9].
There is a lack of literature on indirect decompression in uniportal endoscopic posterolateral transforaminal lumbar interbody fusion (EPTLIF). Our aim is to evaluate the dimensions of the spinal canal and contralateral foramen before and after EPTLIF, allowing direct assessment of the viability of indirect decompression using this technique.
This is a retrospective study of all patients who underwent EPTLIF in a tertiary spine centre by a single fellowship-trained surgeon from 2020 to 2022. The inclusion criteria are patients who underwent EPTLIF for degenerative lumbar conditions such as lumbar spinal stenosis and spondylolisthesis with dynamic instability, without any contralateral radiculopathy. Cases who had previous spinal surgeries, spinal trauma, suspected spinal malignancies, inflammatory spinal conditions and spinal infection were excluded from this study.
All patients underwent a preoperative radiographs and magnetic resonance imaging (MRI) scans of the lumbar spine for preoperative assessment. Patients with corresponding MRIs and sufficient indications for lumbar fusion were counselled for surgery. The steps and techniques for surgery was as described in a technique paper published previously [10]. Decision for bilateral foraminal decompression, ipsilateral foraminal decompression only or was performed based on the clinician’s judgement and the patient’s symptoms on presentation. Patients who presented with ipsilateral claudication with concordant MRI finding of severe bilateral lateral recess and foraminal stenosis underwent bilateral foraminal decompression EPTLIF. Patients who presented with ipsilateral claudication with concordant MRI of severe ipsilateral severe lateral recess and foraminal stenosis underwent ipsilateral decompression only EPTLIF. Patients who presented with ipsilateral claudication with concordant foraminal stenosis, spondylolisthesis and degenerative disc disease without significant central spinal canal stenosis underwent cage insertion alone without any decompression (no dural decompression was performed, the ligamentum flavum was preserved).
Demographic parameters including patient’s age, sex, and the level of fusion were recorded. Clinical parameters such as the visual analogue scale (VAS) score, the Oswestry Disability Index (ODI) and the McNab criteria were measured at the preoperative review and postoperative review at 1 week, 6 months, and at the final follow-up. Additional MRI scans were also performed at the 1-day postoperative mark and at the final follow-up. Computed tomography scans were also performed at the 1-year mark for assessment of fusion at the level operated.
The preoperative and the 2 postoperative MRIs were reviewed in detail. The cross-sectional area of the spinal canal was measured from the axial cuts parallel to the adjacent end plates at the level of the disc fused. The cross-sectional area of the contralateral foramen was measured from the parasagittal cuts at the centre of the contralateral pedicle at the adjacent levels.
EPTLIF had been described by Wu et al. [10]. We docked at the uniportal stenosis endoscope at laminofacet junction. After identification of the superomedial aspect of the inferior articular facet is often medial and deep to the midpoint of the bony arch forms from the ipsilateral spinolaminar junction of the cephalad lamina to the most inferomedial rounded edge of the inferior articular process (IAP) and the superolateral edge of the inferior articular facet which articulates the superolateral edge of the superior articular facet, we perform complete resection of the inferioer articular facet joint by drilling obliquely upwards and laterally from inferomedial rounded edge of the IAP to superolateral edge of the inferior articular facet for complete resection of inferior articular process. Once IAP is resected, ipsilateral superior articular process is removed. Ipsilateral ligamentum flavum overlying the disc space is removed. Care is taken to preserve the ligamentum flavum overlying the ipsilateral traversing nerve root as well as contralateral ligamentum flavum. Disc space is exposed after hemostasis, traversing nerve root protected by working retractor. We performed endplate preparation under endoscopic guidance and insert 3-dimensional (3D)-printed titanium cage with autograft harvested from the facet joint through the single portal under fluoroscopic guidance with the nerve roots protected by Harrison’s cage glider. Upon completion of cage insertion, percutaneous pedicle screws and rods are inserted to stabilize the fusion segment. In this technique, there is decreased risk in traversing nerve root injury as it is protected by ligamentum flavum and retractor tube (Fig. 1A, B).
Similar steps to EPTLIF with facet resection with cage alone insertion without decompression are taken initially to remove facet. In edition for unilateral decompression, we drill the insertion of the ipsilateral ligamentum flavum at the proximal and distal insertion. We removed the ipsilateral flavum completely to expose ipsilateral traversing nerve root and disc endplate preparation and cage insertion is similar to EPTILIF with cage alone cohort (Fig. 1C, D). Contralateral ligamentum flavum is preserved.
In addition to the steps in EPTLIF with unilateral decompression. We perform lumbar endoscopic unilateral laminotomy with bilateral decompression with over-the-top decompression of contralateral ligamentum flavum and medial tip of superior articular process of the contralateral side to decompress the contralateral lateral recess and the foramen. After bilateral bony decompression is completed, both flava are removed and disc preparation is continued similar to other 2 cohorts of EPTLIF (Fig. 1E, F).
All collected data were tabled using IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA) with statistical significances set at p < 0.05. Baseline characteristics and radiographic parameters as described above for the entire cohort were tabulated and shown in Table 1.
The patient cohort was divided into 3 groups—those who underwent bilateral foraminal decompression, ipsilateral decompression, or cage insertion alone without any decompression. Subgroup analyses were performed within each group, and paired t-test were used for comparison to VAS and ODI at baseline. Changes in the clinical and radiographic parameters were also studied and shown in Table 2.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Nanoori Gangnam Hospital (2022-007) and the national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients had given their informed consent for photographs, videos, and images for publication.
A total of 120 patients were recruited into this study. 152 levels of fusion were performed. The average age was 65.2 years. The mean follow-up period was 13.6 months. The most fused level was L4/L5 followed by L3/L4. There were 46 males and 106 females in the cohort. There was no significant difference between the groups when tested using chi-square test (Table 1). There are 8 cases of spinal stenosis (5%), 112 spondylolisthesis with dynamic instability (73%), 3 prolapsed intervertebral disc (2%), 25 foraminal stenosis (15%), 8 degenerative disc disease (5%).
Looking at the VAS and ODI measurements, there were no significant differences among the 3 groups at baseline. In the entire cohort as well as within the subgroups, there were significant improvements in the VAS as well as the ODI measurements at 1-week postoperation with continued trends of improvement at 6-week postoperation and at the final follow-up (Table 2, Fig. 2).
Looking at the MRI measurements, patients who underwent bilateral decompression had a smaller cross-sectional area of the spinal canal when compared with ipsilateral decompression; and patients who had cage insertion alone without any decompression have the largest cross-sectional area (p < 0.001). There were no significant differences in the contralateral foraminal cross-sectional area among the 3 groups at baseline. In the entire cohort as well as within the subgroups, there were significant improvements in the cross-sectional area of the spinal canal as well as the contralateral foramina at 1-day postoperation and these changes persisted and remained significant at the final follow-up (Table 2, Fig. 3) Bar chart comparing the cross-sectional area of the spinal canal and contralateral foramen are shown in Figs. 4 and 5, respectively.
The authors standardized the width, length and angle of the cages which are 12 mm wide, 28 mm long, and 4° angle cages. There are variable height dimensions depending on the disc height of the patients. We used variable heights of cages with the mean of 11.23–11.52 mm height in the 3 groups. Comparing the 3 cohorts with analysis of variance test, there is no statistical difference in cage height used in the 3 groups. There is statistically significant increment of cross-sectional spinal canal area in bilateral decompression more than ipsilateral decompression which is in term more than nondecompression group at postoperative day one (66.95 ± 58.39, 39.56 ± 47.62, 12.91 ± 34.37 mm2) and final follow-up (134.77 ± 52, 94 ± 40.34, 65.84 ± 33.15 mm2). However, there is no significant changes in contralateral foramen among the 3 groups at postoperative day one and final follow-up (Table 3). There is statistically significant difference improvement of VAS and ODI at final follow-up in bilateral decompression cohort compared to ipsilateral and nondecompression cohort but the difference is clinically insignificant. There was no statistical difference in operative time, contralateral foraminal height at any time point and VAS and ODI at postoperative 1 week in comparison of all 3 groups (Table 3).
Unilateral TLIF has also been shown to cause a contralateral radiculopathy with an incidence of up to 5.3% [11]. This was attributed to undiagnosed contralateral foraminal stenosis, improper noncentral asymmetric cage placements, undersized cages, excessive compression to create lumbar lordosis and a newly herniated disc due to insufficient disc removal and the use of unilateral cages pushing the disc material to the contralateral side [12-15]. Some authors have even recommended for prophylactic decompression of the contralateral side in open cases where foraminal stenosis cannot be visualized without prior decompression. This can be circumvented using endoscopic-assisted techniques which allows direct visualization and assessment of the contralateral foramen. This not only reduces the risks of nerve injury, but allows assessment for contralateral compression and need for foraminotomy [13].
Newer techniques—especially with lateral approach techniques such as lateral lumbar interbody fusion, oblique lumbar interbody fusion (OLIF), and extreme lateral interbody fusion have been shown to achieve sufficient indirect decompression for foraminal stenosis [16]. Indirect decompression can be divided into segmental procedures and global spinal alignment procedures. Examples of segmental procedures include disc space utilization procedures (interbody cages), posterior segment distraction procedures (interspinous devices); and ligamentotaxis techniques to prevent infolding of ligaments that causes compression. With a clearer understanding of the anatomy of the lumbar spine, indirect decompression can be achieved while minimizing the soft tissue and bony injuries that lead to adjacent segment disease.
Castellvi et al. [17] showed that indirect decompression of lumbar spinal stenosis can be achieved using with the lateral transpsoas interbody cages and percutaneous posterior instrumentation, resulting in increases in disc height, foraminal area, and canal area measured immediately postoperatively and were sustained at 1-year postoperation. VAS and ODI scores also showed corresponding improvements that were sustained at 1-year postoperation. Tseng et al. [18] utilized OLIF for treatment of lumbar foraminal stenosis and showed that patients who did not underwent posterior decompression had better back pain VAS scores and ODI scores compared to those who underwent open posterior decompression at 12 months and 24 months postoperatively although there were no significant differences in disc height and foraminal height between the 2 groups. They suggested that the use of interbody cages and posterior instrumentation were sufficient for relieving symptoms in patients with lumbar foraminal stenosis and additional direct posterior decompression may deteriorate results in the follow-up period. Gajjar et al. [19] showed further that severe degenerative lumbar central canal stenosis of Schizas grade C or D can be decompressed indirectly using OLIF. Rao et al. [20] also showed significant indirect foraminal decompression based on the new pedicle-to-pedicle technique in anterior lumbar interbody fusions (ALIF) with up to a 67% increased cross-sectional area of the foraminal dimensions and that the posterior disc height correlated significantly with foraminal height for decompression purposes. Kim et al. [21] analysed MRIs of patients who underwent unilateral MIS TLIF procedures pursuing indirect decompression of the contralateral foramen using cage distraction resulted in increased quantitative and qualitative dimensions of the spinal canal and the contralateral foramen.
Based on our results, this is the first time that such an effect can be seen in unilateral EPTLIF using similar cage techniques (in terms of cage sizing and placement) as well as not aggressively chasing the creation of lumbar lordosis. This becomes a balance of indirect decompression of the neural elements with obtaining sufficient correction of the sagittal imbalance. At the immediate postoperative MRI scans, there was a significant increase in the cross-sectional area of the spinal canal and the contralateral foramen regardless of whether any decompression was performed at all. The improvements in the radiological parameters were not only maintained but continues to improve with time due to subsequent remodeling as shown in the MRI scans at the final follow-up. There is significant change increment in axial cross-sectional cut in final follow-up compared to postoperative day one. In bilateral decompression, the axial cross-sectional area doubled in final follow-up. In ipsilateral decompression group, the cross-sectional area almost tripled in final follow-up and there is 5× increment in nondecompression group due to remodeling of spinal canal after EPTLIF. While foraminal height also remodeled with an average of 1.5× increment across all 3 groups. This remodeling pattern is concordant to the corresponding pattern in the patient-reported VAS and ODI scores at 1-week postoperation with continued trends of improvement at 6-week postoperation and at the final follow-up. Therefore, this technique can be used for indirect decompression of the central canal stenosis and contralateral foraminal stenosis and thus, able to reap the benefit of MIS approaches to preserve the anatomy of the spine and reduce the risk of adjacent segment disease while simultaneously avoiding iatrogenic injuries to the neural elements, dural tears, and epidural haematoma. This approach also allows for direct visualization of the contralateral foramen should the need arise and there are changes in neuromonitoring after the insertion of the interbody cage.
While there are studies on remodeling of spinal canal with the induced change of ligamentum flavum in ALIF [22], and oblique lateral lumbar interbody fusion [23], there is limited literature on remodeling after EPTLIF and other fusion methods, however our findings may provide an insight why patient generally feels better as the follow-up continued over a period of time as spinal canal area gets wider over time due to remodeling. More studies of such findings are required to understand this concept of spinal canal remodeling after fusion surgery.
In patients presented predominantly with claudication with minimal back pain, endoscopic foraminotomy is an alternative, minimally invasive technique compared to fusion designed to deal with foraminal stenosis [24]. However, our cohort of patients had presented with back pain and claudication, we performed EPTLIF to our cohort of patients.
One of the potential advantages of ipsilateral decompression or no decompression alone over bilateral decompression is the reduction of operative time. However, we did not find any statistical difference in surgical timing in our comparison of the 3 groups. This demonstrates the complexity of EPTLIF as a multiple step procedure which additional of 5–20 more minutes of additional bilateral decompression may not significantly add more surgical time overall. Although we find a statistical difference in final VAS and ODI with better clinical results in bilateral decompression compared to ipsilateral and no decompression, there is no clinically significant difference as the difference is minor. We suggest to perform bilateral decompression when MRI demonstrated severe central and bilateral lateral recess and foraminal stenosis and ipsilateral or no decompression in patients with MRI demonstrates predominantly ipsilateral degenerative changes.
Uniquely, looking at the cohort who had cage insertion without any dural decompression, there is a significant improvement in both patient-reported outcomes (VAS and ODI scores) as well as radiographic parameters (cross-sectional area of the spinal canal and the contralateral foramen). Such indirect decompression result has only been previously shown in anterior and lateral approach techniques and this is the first time it has been reported in posterior approach endoscopic techniques. This is due to the possibility of using a large cage for indirect disc height restoration with this technique. Direct visualization of the end plate for denudation and the use of a large 3D-printed cage also allowed a high rate of fusion contributing to the maintenance of the intervertebral space stability and resulting in the subsequent remodeling and enlargement of the radiographic parameters recorded. The use of large cages also allows greater lordosis to be achieved especially in the lower lumbar segments thus, improve the lordosis distribution index and reduce the risks of revision surgery in the future [25].
There is an increasing interest in 3D-printed titanium cages in lumbar spinal fusion. We used 3D-printed titanium cages in our cohort of patients. One of the limitations of minimally invasive fusion is cage subsidence, the use of 3D-printed cage was associated with lower rates of subsidence [26]. Kim et al. [27] found that while there is no significant differences in overall fusion rate between PEEK and 3D-printed titanium cages, fusion grade was better in 3D-printed titanium cages. In our technique we performed fusion with straight cages. Choi et al. [28] found that straight cages have lower subsidence rate compared to banana cages possibly due to more medial final position in banana cages. We felt that in EPTLIF, endoscopic direct visualization when we removed intervertebral disc, careful endplate preparation made with blunt endoscopic penfeel, and constant irrigation of inflammatory disc fragments with saline irrigation help in preparation of endplate without significant endplate violation to avoid subsidence. Together with 3D-printed cages potentially lower rate of subsidence, there is potential for better fusion rate. However, as our study is focus on MRI evaluation of spinal canal parameters, we did not evaluate further on fusion rate and subsidence which would be of academic interest in our future studies. Overall, minimally invasive transforaminal interbody fusion such as EPTLIF has promising results and potential in being treatment of choice for fusion with better understanding of technique and technology of interbody cages [29].
There are a few limitations of this study. The surgeon was not blinded to the patient’s symptoms and were given the option to decide if the patient requires bilateral decompression, ipsilateral decompression or cage insertion alone without any decompression during the surgery. At subsequent reviews, both the patient and the surgeon were not blinded as well when recording the patient-reported outcomes. Variabilities in measurements of the cross-sectional area of the spinal canal and the contralateral foramen is inescapable due to human error. Majority of our patients presented with spondylolisthesis, there is limitation in finding a difference during analysis in central and foraminal expansion between spondylolisthesis and spinal stenosis group. Our study is predominantly an MRI study, fusion rate and subsidence are not reflected in the study. Lastly, we did not include patients with bilateral lower limb radiculopathy that may benefit from bilateral decompressions. Future avenues of study could focus on patients with not only sagittal imbalance but also coronal deformity, as well as patients who underwent 3 or more level surgery.
Indirect decompression of both the spinal canal and the contralateral foramen can be achieved via EPTLIF. This radiological finding is supported by patient-reported outcome scores. Initial improvement (immediate postoperation) is not only maintained at the final follow-up, but there is continued improvement due to subsequent remodeling. Decompression on an asymptomatic contralateral side is not necessary unless it is accompanied by a very severe spinal stenosis due to the increased risks of injury in contralateral decompression.
NOTES
Conflict of Interest
Dr. Pang Hung Wu as first co-author declared his spouse is the director of Singapore based company Endocare PTE Ltd. which distributes orthopaedic and spine products including BESS, NSK drill, and Bonss energy system. No other co-authors have conflict of interest.
ACKNOWLEDGEMENTS
We would like to acknowledge scientific team members Ms. So-Jung Yoon, Se-Won Lee, and Mr. Kyeong Rae Kim for providing assistance in statistical support, acquiring full text articles and managing digital works.
Fig. 1.
Cartoon and intraoperative picture demonstrating the 3 types of technique of EPTLIF. (A) Cartoon demonstrated EPTLIF with cage insertion and conservation of ipsilateral ligamentum flavum overlying traversing nerve root and contralateral ligamentum flavum (dotted yellow arrow). (B) Intraoperative picture demonstrated interbody cage placed lateral to ipsilateral ligamentum flavum. (C) Cartoon demonstrated EPTLIF with cage insertion and complete removal of ipsilateral ligamentum flavum overlying traversing nerve root while preserving the contralateral ligamentum flavum (dotted red arrow). (D) Intraoperative picture demonstrated interbody cage placed lateral to ipsilateral traversing nerve root with ligamentum flavum removed. (E) Cartoon demonstrated EPTLIF with cage insertion and complete removal of both ligamentum flava (dotted red and blue arrows). (F) Intraoperative picture demonstrated contralateral decompression with removal of contralateral ligamentum flavum prior to placement of interbody cage on the ipsilateral side. EPTLIF, endoscopic posterolateral transforaminal lumbar interbody fusion.
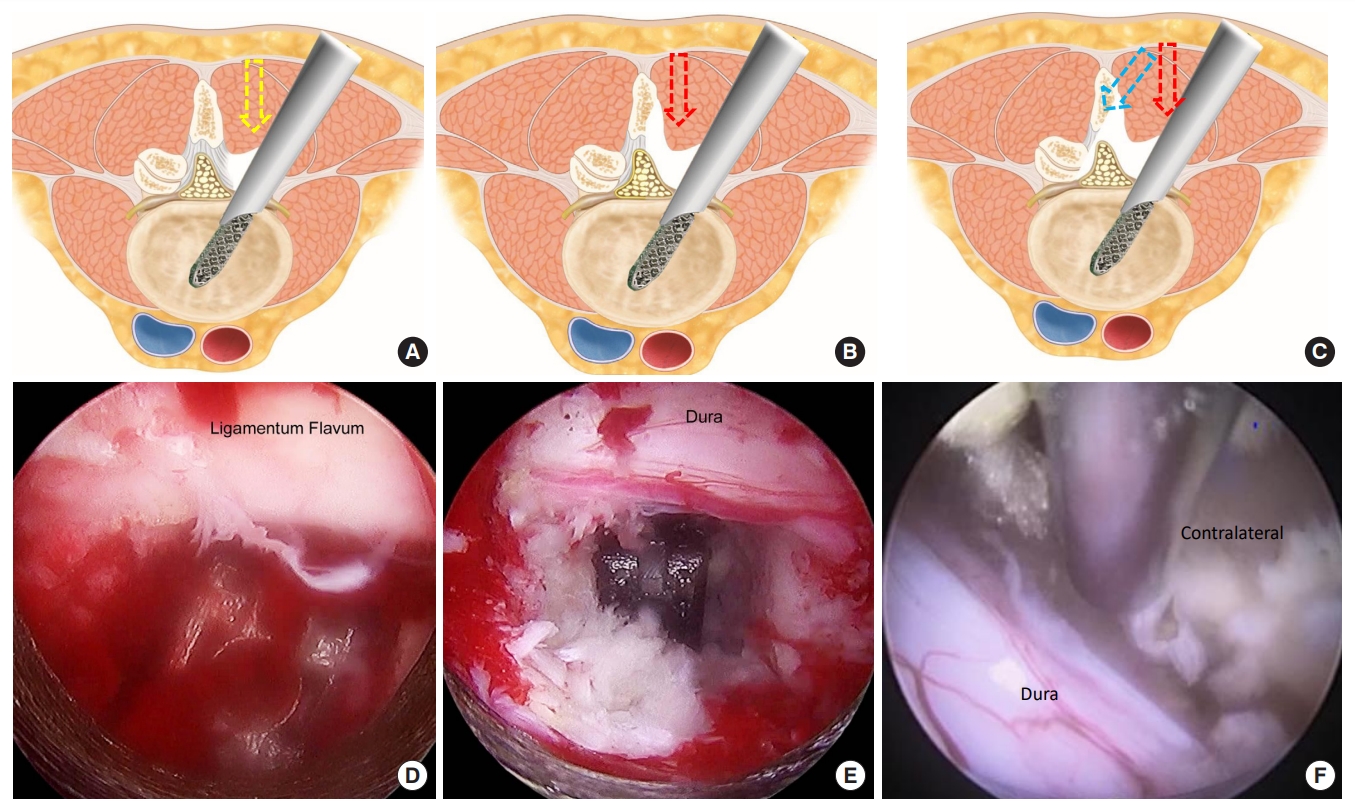
Fig. 2.
(From left to right) Preoperative baseline magnetic resonance imaging (MRI), 1-day postoperative MRI, and MRI scans at the final follow-up of a patient who underwent endoscopic posterolateral transforaminal lumbar interbody fusion.
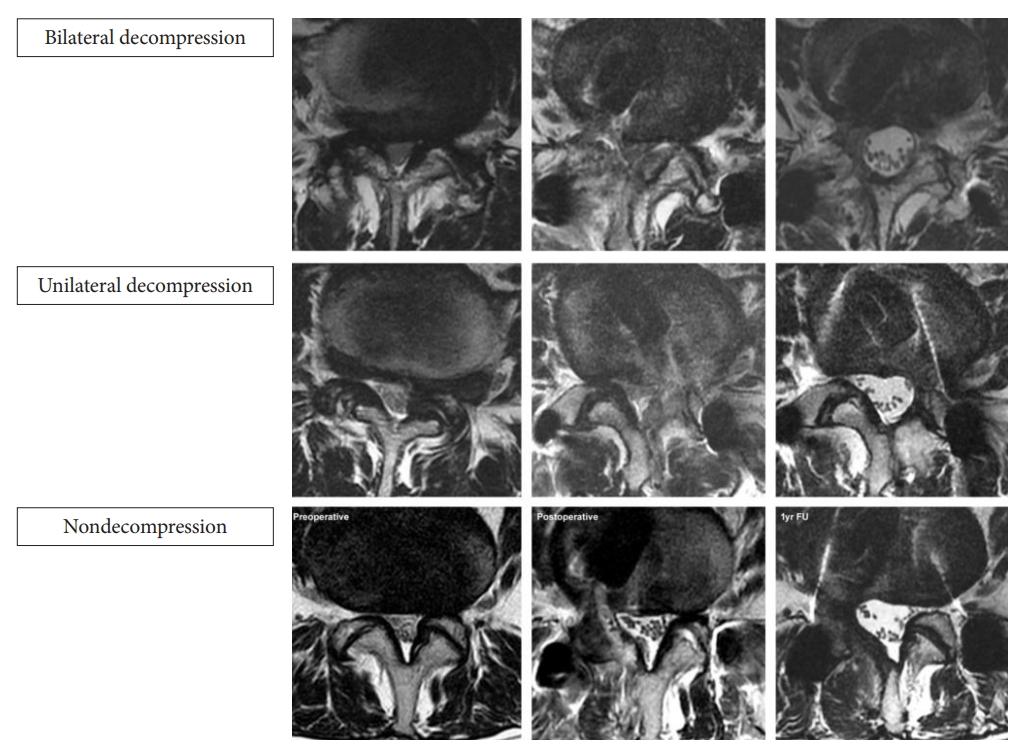
Fig. 3.
(From left to right) Preoperative baseline, 1-day postoperative, and final follow-up axial and right parasagittal MRI in nondecompression left EPTLIF of L4/5. EPTLIF, endoscopic posterolateral transforaminal lumbar interbody fusion.
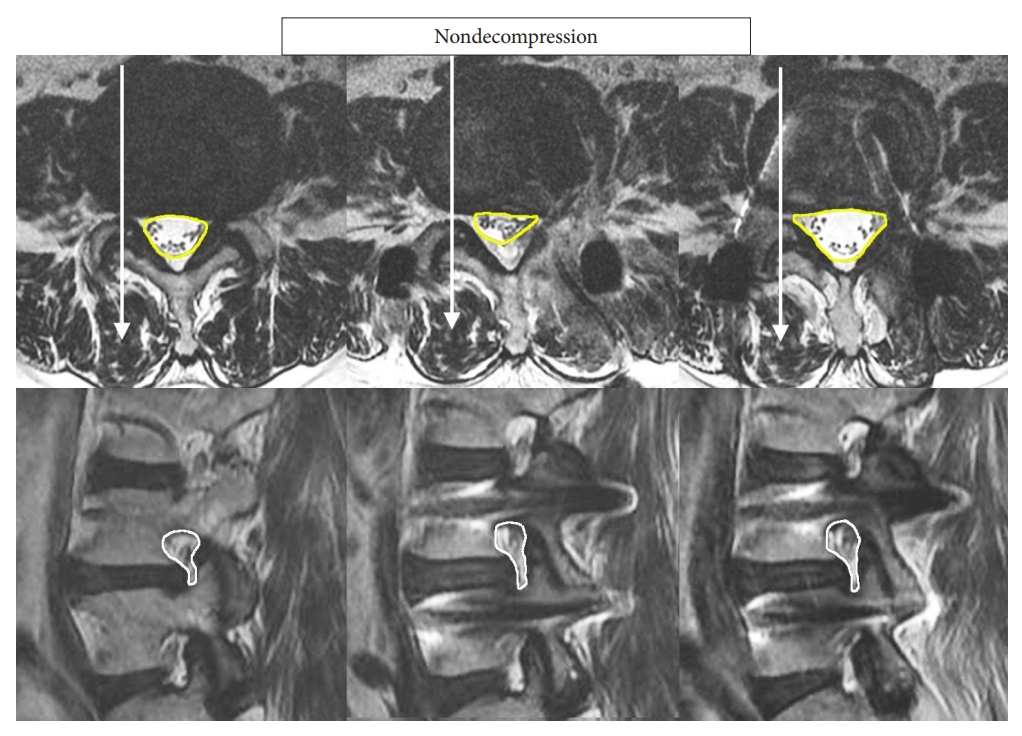
Fig. 4.
Bar chart comparing the cross-sectional area of the spinal canal at preoperative baseline, 1-day postoperation, and at final follow-up. *p < 0.001, significant difference when compared to preoperative baseline.
Fig. 5.
Bar chart comparing the cross-sectional area of the contralateral foramen at preoperative baseline, 1-day postoperation, and at final follow-up. *p < 0.001, significant difference when compared to preoperative baseline.
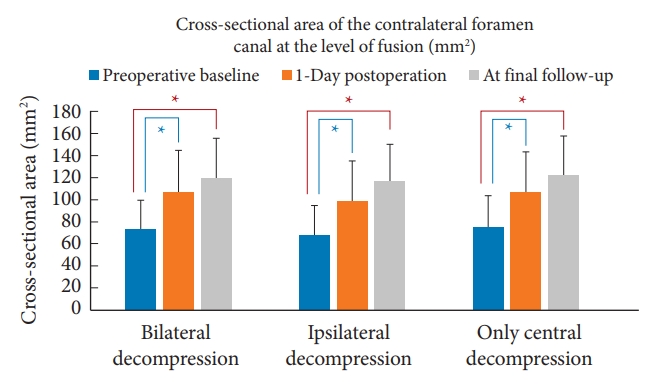
Table 1.
Baseline demographic data, clinical parameters and radiographic measurements
Table 2.
Subgroup analyses looking at clinical parameters and radiographic measurements at baseline and on subsequent follow-up
| Variable | Bilateral decompression | Ipsilateral decompression | Cage insertion alone without any decompression | |
|---|---|---|---|---|
| VAS measurements | ||||
| Preoperative baseline | 8.0 ± 1.2 | 7.5 ± 1.3 | 7.6 ± 1.4 | |
| 1-Week postoperation | 3.3 ± 0.6* | 3.5 ± 0.7* | 3.4 ± 0.7* | |
| 6-Month postoperation | 2.4 ± 0.7* | 2.7 ± 0.8* | 2.6 ± 0.9* | |
| At final follow-up | 1.9 ± 0.8* | 2.2 ± 0.8* | 2.2 ± 0.9* | |
| ODI | ||||
| Preoperative baseline | 73.6 ± 6.9 | 70.0 ± 9.4 | 70.5 ± 9.4 | |
| 1-Week postoperation | 32.7 ± 4.2* | 33.6 ± 7.1* | 32.9 ± 5.7* | |
| 6-Month postoperation | 27.1 ± 3.8* | 28.7 ± 5.2* | 27.8 ± 5.6* | |
| At final follow-up | 24.1 ± 4.5* | 26.0 ± 4.7* | 25.4 ± 5.2* | |
| Cross-sectional area of the spinal canal (mm2) | ||||
| Preoperative baseline | 57.0 ± 38.0 | 89.3 ± 63.2 | 138.3 ± 65.1 | |
| 1-Day postoperation | 123.9 ± 63.3* | 128.9 ± 47.0* | 151.2 ± 65.6* | |
| At final follow-up | 191.8 ± 51.2* | 183.3 ± 44.9* | 204.1 ± 67.0* | |
| Cross-sectional area of the contralateral foramen (mm2) | ||||
| Preoperative baseline | 73.3 ± 26.6 | 69.5 ± 26.4 | 75.1 ± 28.8 | |
| 1-Day postoperation | 106.4 ± 38.2* | 99.0 ± 36.3* | 106.5 ± 36.8* | |
| At final follow-up | 120.4 ± 35.5* | 116.9 ± 33.6* | 122.9 ± 34.8* | |
Table 3.
ANOVA test comparison of EPTLIF with bilateral decompression, ipsilateral decompression, and cage insertion without any decompression
| Variable | Bilateral decompression | Ipsilateral decompression | Cage insertion alone without any decompression | p-value |
|---|---|---|---|---|
| Height of cage used in the surgery (mm) | 11.37 ± 1.26 | 11.52 ± 1.33 | 11.23 ± 1.56 | 0.558 |
| Surgical timing of cage insertion (min) | 139.29 ± 19.37 | 134.41 ± 16.86 | 137.33 ± 20.67 | 0.534 |
| POD 1 MRI axial cut cross-sectional area increment of spinal canal area in (POD 1–Preop) (mm2) | 66.95 ± 58.39 | 39.56 ± 47.62 | 12.91 ± 34.37 | < 0.001* |
| POD final MRI axial cut cross-sectional area increment of spinal canal area in (POD final–Preop) (mm2) | 134.77 ± 52 | 94 ± 40.34 | 65.84 ± 33.15 | < 0.001* |
| POD 1 MRI sagittal cut increment of foraminal area of contralateral foramen (POD1–Preop) (mm2) | 33.07 ± 28.69 | 29.54 ± 29.04 | 31.31 ± 25.94 | 0.853 |
| POD final MRI sagittal cut increment of foraminal area of contralateral foramen (POD final–Preop) (mm2) | 47.12 ± 27.19 | 47.4 ± 28.81 | 47.77 ± 31.86 | 0.994 |
| POD 1 week improvement of VAS (Preop–POD 1 VAS) | 4.63 ± 1.4 | 3.95 ± 1.48 | 4.16 ± 1.39 | 0.106 |
| POD 6 months improvement of VAS (Preop–POD 6 months VAS) | 5.54 ± 1.52 | 4.79 ± 1.26 | 5.03 ± 1.42 | 0.059 |
| POD final improvement of VAS (Preop–POD final VAS) | 6.06 ± 1.47 | 5.24 ± 1.32 | 5.41 ± 1.57 | 0.042* |
| POD 1 week improvement of ODI (Preop–POD 1 ODI) | 40.86 ± 8.24 | 36.38 ± 11.52 | 37.6 ± 10.26 | 0.145 |
| POD 6 months improvement of ODI (Preop–POD 6 months ODI) | 46.51 ± 8.18 | 41.29 ± 8.94 | 42.72 ± 9.38 | 0.035* |
| POD final improvement of ODI (Preop–POD final ODI) | 49.49 ± 8.94 | 43.95 ± 8.84 | 45.11 ± 10.04 | 0.028* |
REFERENCES
1. Fritzell P, Hägg O, Wessberg P, et al. 2001 Volvo Award Winner in Clinical Studies: lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine (Phila Pa 1976) 2001;26:2521-32. discussion 2532-4.

2. Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am 2009;91:1295-304.


3. Ahmed SI, Javed G, Bareeqa SB, et al. Comparison of decompression alone versus decompression with fusion for stenotic lumbar spine: a systematic review and meta-analysis. Cureus 2018;10:e3135.



4. Yee TJ, Terman SW, La Marca F, et al. Comparison of adjacent segment disease after minimally invasive or open transforaminal lumbar interbody fusion. J Clin Neurosci 2014;21:1796-801.


5. Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J 2004;4(6 Suppl):190S-194S.


6. Harms J. Dorsale Repositionsspondylodese bei lumbalen Spondylolisthesis. Oper Orthop Traumatol 1999;11:79.


7. Lai PL, Chen LH, Niu CC, et al. Relation between laminectomy and development of adjacent segment instability after lumbar fusion with pedicle fixation. Spine (Phila Pa 1976) 2004;29:2527-32. discussion 2532.


8. Pourtaheri S, Issa K, Lord E, et al. Paraspinal muscle atrophy after lumbar spine surgery. Orthopedics 2016;39:e209-14.


9. Epstein NE. More nerve root injuries occur with minimally invasive lumbar surgery, especially extreme lateral interbody fusion: a review. Surg Neurol Int 2016;7(Suppl 3):S83-95.



10. Wu PH, Kim HS, Lee YJ, et al. Uniportal full endoscopic posterolateral transforaminal lumbar interbody fusion with endoscopic disc drilling preparation technique for symptomatic foraminal stenosis secondary to severe collapsed disc space: a clinical and computer tomographic study with technical note. Brain Sci 2020;10:373.



11. Chen YL, Hu XD, Wang Y, et al. Contralateral radiculopathy after unilateral transforaminal lumbar interbody fusion: causes and prevention. J Int Med Res 2021;49:3000605211037475.



12. Hu HT, Ren L, Sun XZ, et al. Contralateral radiculopathy after transforaminal lumbar interbody fusion in the treatment of lumbar degenerative diseases: a case series. Medicine (Baltimore) 2018;97:e0469.


13. Yang Y, Liu ZY, Zhang LM, et al. Risk factor of contralateral radiculopathy following microendoscopy-assisted minimally invasive transforaminal lumbar interbody fusion. Eur Spine J 2018;27:1925-32.



14. Hwang SH, Park SW, Kim YB. Risk factors for symptomatic contralateral foraminal stenosis after unilateral transforaminal lumbar interbody fusion. World Neurosurg 2020;133:e452-8.


15. Cho PG, Park SH, Kim KN, et al. A morphometric analysis of contralateral neural foramen in TLIF. Eur Spine J 2015;24:783-90.



17. Castellvi AE, Nienke TW, Marulanda GA, et al. Indirect decompression of lumbar stenosis with transpsoas interbody cages and percutaneous posterior instrumentation. Clin Orthop Relat Res 2014;472:1784-91.



18. Tseng SC, Lin YH, Wu YC, et al. Indirect decompression via oblique lumbar interbody fusion is sufficient for treatment of lumbar foraminal stenosis. Front Surg 2022;9:911514.



19. Gajjar S, Jhala A, Mistry M. Effectiveness of indirect decompression in severe degenerative lumbar central canal stenosis by oblique lumbar interbody fusion. J Minim Invasive Spine Surg Tech 2021;6:131-40.
20. Rao PJ, Maharaj MM, Phan K, et al. Indirect foraminal decompression after anterior lumbar interbody fusion: a prospective radiographic study using a new pedicle-to-pedicle technique. Spine J 2015;15:817-24.


21. Kim MC, Park JU, Kim WC, et al. Can unilateral-approach minimally invasive transforaminal lumbar interbody fusion attain indirect contralateral decompression? A preliminary report of 66 MRI analysis. Eur Spine J 2014;23:1144-9.



22. Ohtori S, Orita S, Yamauchi K, et al. Change of lumbar ligamentum flavum after indirect decompression using anterior lumbar interbody fusion. Asian Spine J 2017;11:105-12.




23. Mahatthanatrakul A, Kim HS, Lin GX, et al. Decreasing thickness and remodeling of ligamentum flavum after oblique lumbar interbody fusion. Neuroradiology 2020;62:971-8.



24. Giordan E, Billeci D, Del Verme J, et al. Endoscopic transforaminal lumbar foraminotomy: a systematic review and meta-analysis. Pain Ther 2021;10:1481-95.




25. Bari TJ, Heegaard M, Bech-Azeddine R, et al. Lordosis distribution index in short-segment lumbar spine fusion - can ideal lordosis reduce revision surgery and iatrogenic deformity? Neurospine 2021;18:543-53.




26. Toop N, Dhaliwal J, Grossbach A, et al. Subsidence rates associated with porous 3D-printed versus solid titanium cages in transforaminal lumbar interbody fusion. Global Spine J 2023 Feb 14:21925682231157762doi: 10.1177/21925682231157762. [Epub].



27. Kim DY, Kwon OH, Park JY. Comparison between 3-dimensional-printed titanium and polyetheretherketone cages: 1-year outcome after minimally invasive transforaminal interbody fusion. Neurospine 2022;19:524-32.





- TOOLS
-
METRICS

-
- 1 Crossref
- Scopus
- 2,620 View
- 172 Download


























